Question #4f48c
1 Answer
A) Boiling point; B) add
Explanation:
A) Rubbing alcohol and water
The quickest way to differentiate between rubbing alcohol and water is to smell it. Rubbing alcohol has a characteristic strong odour similar to that of ethanol.
I would call this a physical property, but others call it a chemical property because of chemical changes that may occur in the smell receptors in the nose.
However, the boiling point is definitely a physical property.
The boiling point of isopropyl alcohol is 83 °C; the boiling point of water is 100 °C.

B) Saltwater and water
Add a few drops of a silver nitrate solution to each liquid.
The saltwater will form a white precipitate; the water will not form a precipitate.
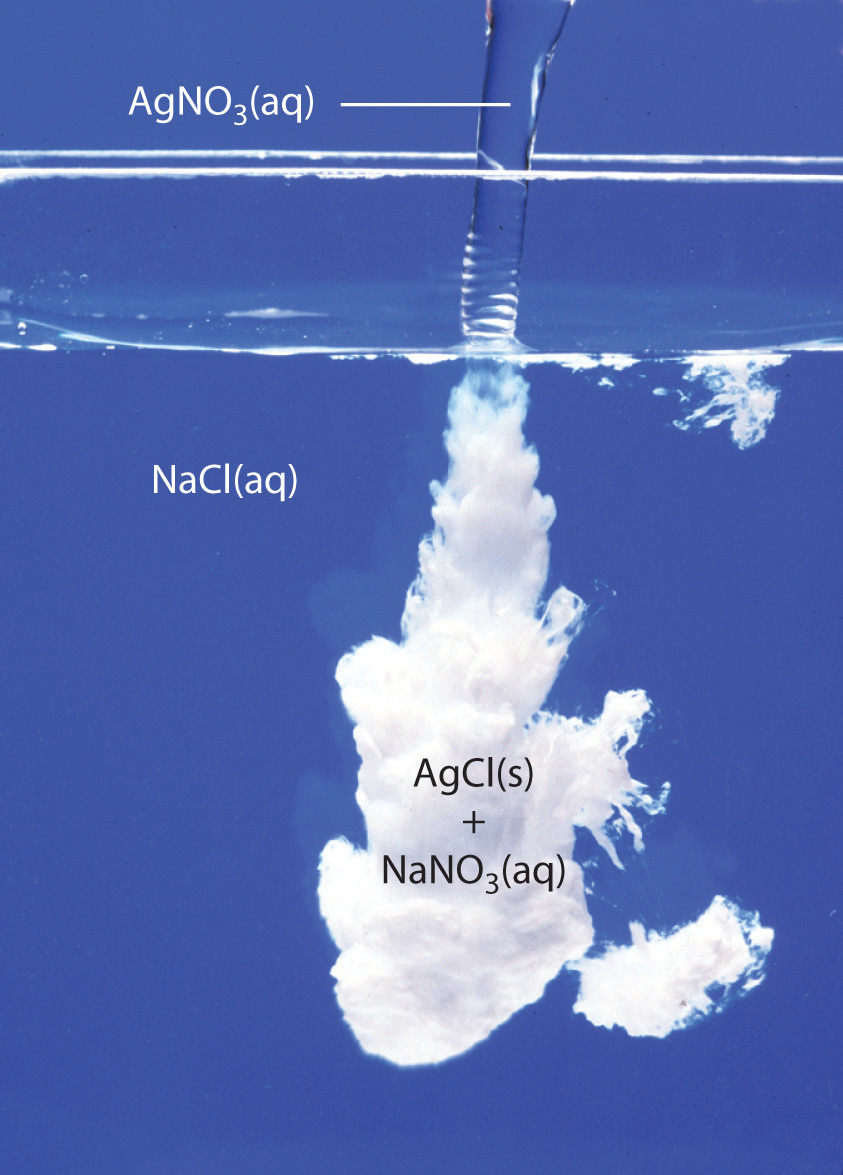
The formation of a precipitate is a chemical property.
C) Carbonated water
Add 1 mL of the liquid to lime water (a saturated solution of calcium hydroxide).
The carbonated water will give a cloudy white precipitate. The water will give no reaction.

The formation of a precipitate is a chemical property.

