Question #f3813
1 Answer
Both can have polar bonds
Explanation:
The conventional explanation is based on the definition of polar molecule, that is a molecule with a net or permanent dipole momentum. The electrical dipole is an uneven distribution of electrons and nuclei, resulting in unbalanced or positive and negative ends.
A polar bond, due to a marked difference in electronegativity between the bonded atoms, corresponds to a dipole vector and is the most common source of the polarity of the whole molecule.
In other words, molecules without polar bonds are usually apolar.
If a small molecule such as O=C=O contains polar bonds arranged symmetrically, the opposite dipole vectors cancel out each other.
So, we conclude that polar molecules and small non-polar molecules can have one similarity in that both have polar bonds.
Azulene is one exception:
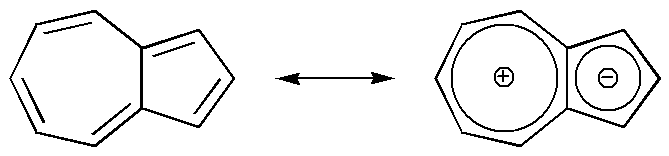
It owes its beautiful deep blue colour to a strong dipole even though it is formed from C-C and C-H apolar bonds as the other hydrocarbons.
This odd case remembers to us that the polarity of a molecule can also emerge as a large-scale molecular effect, caused by a particular complexity of the molecule, and not as a simple nonzero sum of dipole vectors, even in the frame of the conventional definition of polarity.
The following is a non-conventional explanation, based on a practical definition of polarity.
If we (practically) define polarity of a molecule as its enhanced aptitude to interact with external molecules (e.g. water molecules), then completely symmetric molecules as phloroglucinol

and dioxane
are actually polar molecules, i.e. partially and completely water soluble. For large molecules, we can overlook the criterion of symmetry and the overall molecular dipole momentum.
This means that polar o dipole-dipole interactions are "uninterested" to the net dipole of the whole molecule, but they occur "locally", in the sense that any polar part of the molecule can as well interact with any external dipoles even though the molecule is symmetrical and apolar from the conventional point of view.

