A 12.0 L gas cylinder has been filled with 5.40 moles of gas. You measure the pressure to be 3.10 atm What is the temperature inside the tank?
1 Answer
Aug 18, 2016
The temperature inside the tank is
Explanation:
Since we are given the number of moles, pressure, and volume of gas, we have to use the ideal gas law equation to determine the temperature.
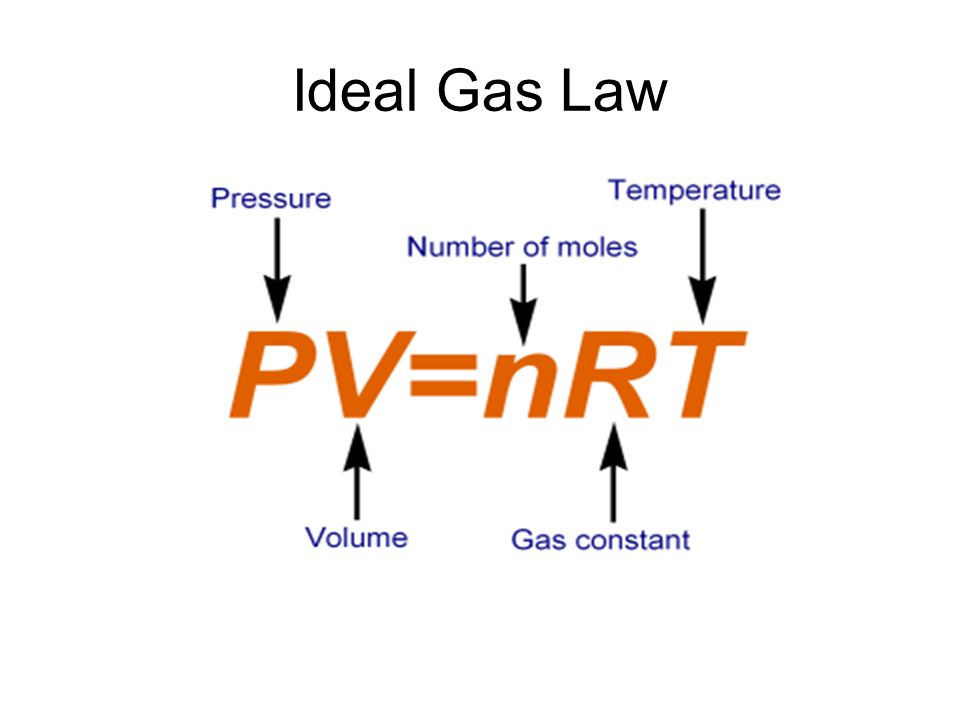
- P can have units of atm, depending on the units of the universal gas constant
- V must have units of liters
- n should have units of moles
- R has a value of 0.0821 with units of
#(Lxxatm)/ (molxxK)# - T has units of Kelvins.
Next, list your known and unknown variables:
Rearrange the ideal gas law to solve for T:

