A 600 K piece of metal would emit radiation at a peak wavelength of what?
1 Answer
Explanation:
For an object that emits black-body radiation, the peak wavelength is simply the wavelength at which the intensity of the emitted radiation is at its maximum value.
Now, black-body radiation is a type of electromagnetic radiation emitted by a black body that's held at a constant temperature. The important thing to realize about black-body radiation is that its intensity depends exclusively on the absolute temperature of the body.
The relationship between the maximum intensity of the black-body radiation emitted by an object and the wavelength at which it occurs is given by the Wien displacement law equation
#color(blue)(lamda_"max" = b/T)" "# , where
In your case, the absolute temperature of the metal is said to be equal to
#lamda_"max" = (2.89777 * 10^(-3) "m" color(red)(cancel(color(black)("K"))))/(600color(red)(cancel(color(black)("K")))) = 4.83 * 10^(-6)"m"#
Expressed in nanometers and rounded to one sig fig, the number of sig figs you have for the temperature of the metal, the answer will be
#lamda_"max" = color(green)("5000 nm")#
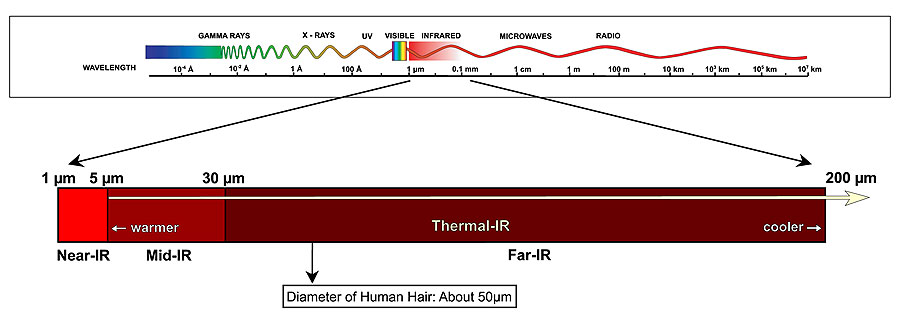
This wavelength places the radiation in the infrared region of the electromagnetic spectrum. More specifically, this wavelength corresponds to the Far-IR region.