A flask contains a gas mixture of methane, hydrogen. and nitrogen with partial pressures of 1 atm. 1.2 atm, and 1.1 aim, respectively. What is the total pressure of the mixture?
1 Answer
Explanation:
The idea here is that you can use Dalton's Law of Partial Pressures to express the total pressure of the gaseous mixture as the sum of the partial pressures of the individual components of the mixture.
#color(blue)(P_"total" = sum_i (P_"partial i")) " "# , where
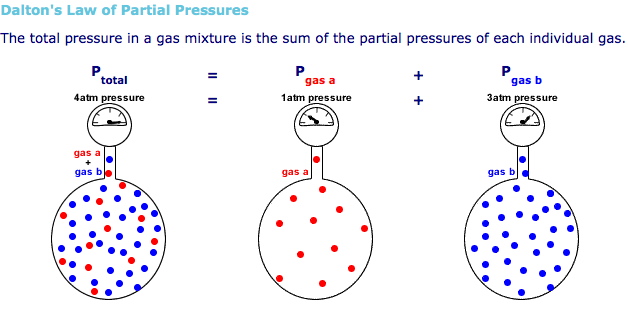
In your case, you know that the mixture contains three gases, methane, hydrogen, and nitrogen, each with its own partial pressure
#"CH"_4 -> "1.0 atm"# #"H"_2 -> "1.2 atm"# #"N"_2 -> "1.1 atm"#
This means that the total pressure of the mixture will be
#P_"total" = P_(CH_4) + P_(H_2) + P_(N_2)#
#P_"total" = "1.0 atm" + "1.2 atm" + "1.1 atm" = color(green)("3.3 atm")#
The underlying principle that's at work here is the fact that at constant temperature and volume, the pressure of a gas is proportional ot the number of moles of gas.

