A container with a volume of #6 L# contains a gas with a temperature of #720^o K#. If the temperature of the gas changes to #420^o K# without any change in pressure, what must the container's new volume be?
1 Answer
Oct 24, 2016
The new volume will be 4 L rounded to one significant figure.
Explanation:
This question involves the use of Charles' law, which states that the volume and temperature are directly proportional as long as pressure and amount are kept constant. This means that if the temperature increases, the volume increases and vice versa.
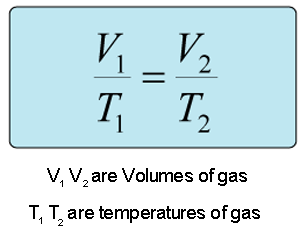
Given
Unknown
Solution
Rearrange the equation to isolate

