A heating element of resistance r is fitted inside an adiabatic cylinder which carries a frictionless piston of mass m and crosssection A as shown in diagram. The cylinder contains one mole of an ideal diatomic gas. The current flows through the element ?
such that the temperature rises with time t as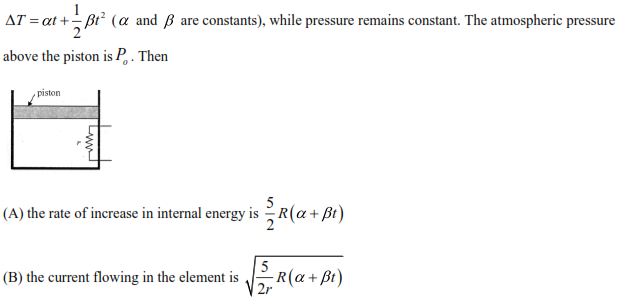
(C) . the piston moves up with constant acceleration .
(D) . the piston moves up with constant velocity.
such that the temperature rises with time t as
1 Answer
Explanation:
Well as the cylinder is adiabatic in nature,it will not release nor accept any heat other than the heat produced by the resistor.
Now.the pressure on the gas is solely due to the atmospheric pressure and weight of the piston,which don't change with the process.
So,it is an isobaric process.
Now,change in internal energy for an isobaric process is given as,
Now,
And,
So,
so,
So,option
Now,we know,power generated due to current flowing
Now,here,
Now,for an isobaric process
So,
or,
So,option
Now,work done in isobaric process is
now,
As,
so,
or,
or,
so,
So,
Compare with
So,the piston will move upward with constant acceleration.
So,option

