A) Identify the amine functional group from the compound? B) Identify the alcohol functional group from the compound?
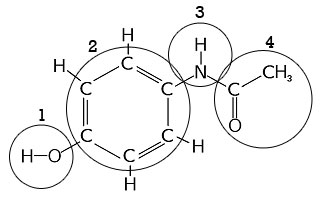
1 Answer
These are just functional groups you'll have to know and memorize.
An amine group is
#-stackrel("H")stackrel(|)"N"-"H"# (primary amine)
#color(white)(.,)ddot#
#-stackrel("R'")stackrel(|)"N"-"H"# (secondary amine)
#color(white)(.,)ddot#
#-stackrel("R'")stackrel(|)"N"-"R''"# (tertiary amine)
#color(white)(.,)ddot#
#-stackrel("R'")(stackrel(|)("N"^(+)))-"R''"# (quaternary ammonium salt)
#color(white)(....)|#
#color(white)(.,)"R'''"#
And an alcohol group could be:
#-stackrel("H")stackrel(|)"C"-ddot"O"-"H"# (primary alcohol)
#" "| color(white)(.-)ddot#
#color(white)(..)"H"#
#-stackrel("R")stackrel(|)"C"-ddot"O"-"H"# (secondary alcohol)
#" "| color(white)(.-)ddot#
#color(white)(..)"H"#
#-stackrel("R")stackrel(|)"C"-ddot"O"-"H"# (tertiary alcohol)
#" "| color(white)(.-)ddot#
#color(white)(..)"R'"#
Of course, all of these bond angles are based on the tetrahedral electron geometry.

