A. Identify the limiting reagent. B. State how many grams of the excess reagent remain. C. State how many grams of product are produced.?
Aspirin (C9H8O4, molecular mass 180.2 g/mol) is synthesized by the
reaction of salicylic acid (C7H6O3, molecular mass 138.1 g/mol) with acetic
anhydride (C4H6O3, molecular mass 102.1 g/mol) according to the reaction
2 C7H6O3 (s) + C4H6O3(l) → 2C9H8O4(s)+ H2O(l)
2.0g of salicylic acid and 5.4 g of acetic anhydride are mixed and the reaction is
allowed to go to completion. Do each of the following:
A. Identify the limiting reagent.
B. State how many grams of the excess reagent remain.
C. State how many grams of product are produced.
Aspirin (C9H8O4, molecular mass 180.2 g/mol) is synthesized by the
reaction of salicylic acid (C7H6O3, molecular mass 138.1 g/mol) with acetic
anhydride (C4H6O3, molecular mass 102.1 g/mol) according to the reaction
2 C7H6O3 (s) + C4H6O3(l) → 2C9H8O4(s)+ H2O(l)
2.0g of salicylic acid and 5.4 g of acetic anhydride are mixed and the reaction is
allowed to go to completion. Do each of the following:
A. Identify the limiting reagent.
B. State how many grams of the excess reagent remain.
C. State how many grams of product are produced.
1 Answer
This is a pretty complex stoichiometry question. I remember answering a similar question on my first general chemistry exam...
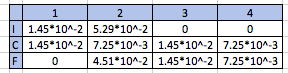
We want to build an ICF chart relating how many moles are produced or reacted in this reaction:
Below the initial reaction, I built the table with the data, from this table we can answer your questions.
(1) The limiting reagent is salicylic acid.
(2) Approximately
(3) Approximately

