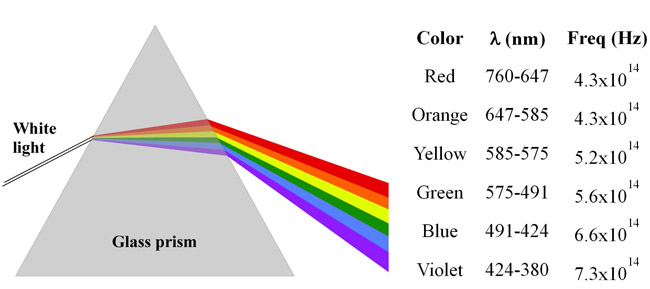
A metal foil has a threshold frequency of 5.45× 1014 Hz. Which of the colors of visible light have enough energy to eject electrons from this metal?
Red
Orange
Yellow
Green
Blue
Indigo
Violet
Red
Orange
Yellow
Green
Blue
Indigo
Violet
1 Answer
Nov 16, 2016
Einstein photoelectric equation
Where
This equation reveals that ejection of electron from metal surface i.e KE of electron is possible iff
Given
As per above table the frequencies of Green,Blue,Indigo and Violet lights are greater than the thresold frequency. So those radiations are capable of ejecting electron from metal surface for having enough energy.

