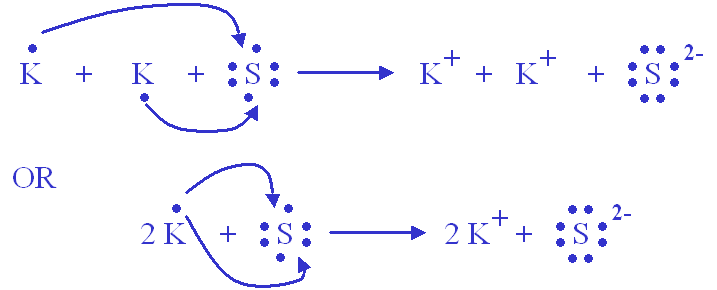A potassium ion has a charge of 1+. A sulfide ion has a charge of 2- .Wha tis the chemical formula for potassium sulfide?
1 Answer
Explanation:
One of the most important things to remember about ionic compounds is that they must be electrically neutral.
This means that the overall positive charge of the cations must be balanced by the overall negative charge of the anions.
In this case, you know that your compound contains potassium cations,
Notice that the cation carries a
So, how would you do that?
How about using two potassium cations to balance one sulfide anion?
The overall charges will be
#2 xx (1+) = (2+) -># coming from two#"K"^(+)# cations#1 xx (2-) = (2-) -># coming from one#"S"^(2-)# anion
This means that the chemical formula for this ionic compound will be
#"K"_2"S"_1 implies color(green)("K"_2"S")#