A research assistant made 160 mg of radioactive sodium (Na^24) and found that there was only 20 mg left 45 h later, what is the half-life of Na^24?
1 Answer
Apr 12, 2017
The half life is
Explanation:
There are 2 ways for solving this problem
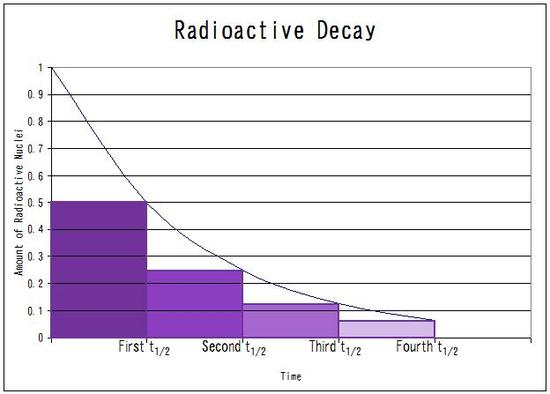
Every half life, the amount is divided by
After
After
After
The half life is
The half life of sodium 24 is
The radioactive decay constant is
We apply the equation
The activity is proportional to the mass.

