A transition of an electron between two different energy levels produced a series of line spectrum . calculate the wavelength of (β) line in Paschen series. wavelength in nanometer?
1 Answer
Jul 6, 2015
I found:
Explanation:
Considering your
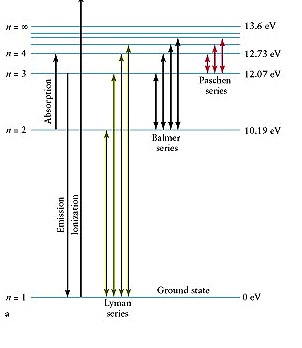
I would use Rydberg Formula:
Where:
so you get:

