A voltaic cell is constructed using the following half-reactions. How do you determine the cell reaction?
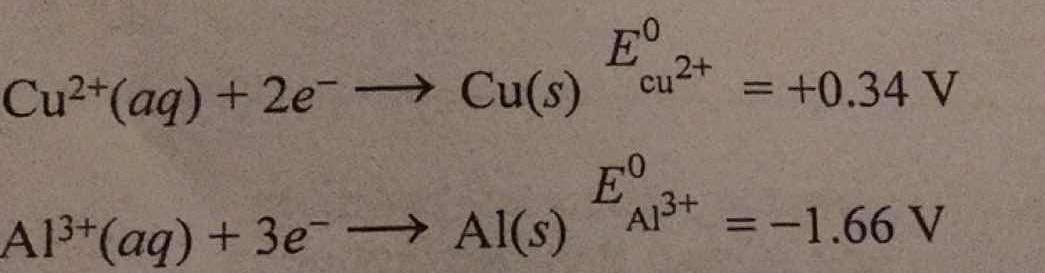
1 Answer
See below.
Warning: Kind of long answer.
Explanation:
The electrons flow through the circuit from the more negative electrode to the less negative electrode so the electrons will flow from the aluminium electrode to the copper.
The aluminium electrode is losing electrons so its reaction will be:
(
The other electrode will have a reaction of:
We multiply equation 1 by 2 and equation 2 by 3 so that the number of
and
Overall equation for the reaction:
Standard cell potential = +1.66V + (+0.34V) = +2.00 V
NB: the positive emf value indicates that the reaction is feasible.
Also, when we multiply the equations to balance the overall reaction, emf values remain the same because emf indicates the probability of the reaction occurring and not the quantity of the species.
Hope this helped :)

