According to the VSEPR theory, which shape is possible for a molecule with the molecular formula of AB3 (where the number of total electron groups is unstated)?
1 Answer
I know of
Explanation:
By shape, I'll assume you mean the molecule's molecular geometry; that is, the arrangement of solely the atoms around the central atom, not the nonbonding electron pairs. We would expect the second element (
1.
Let's start with a group
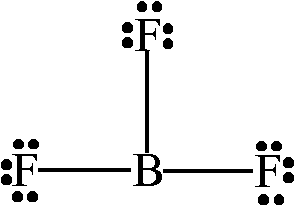
We can see that since there are no nonbonding electrons left on the central boron atom, and there are three bonding pairs, the molecular geometry of
2.
You've probably heard of the compound ammonia,
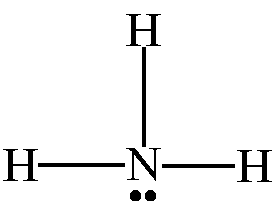
Since
3.
It is possible to form interhalogen compounds; that is, a molecule consisting of only halogen atoms. An example of this is
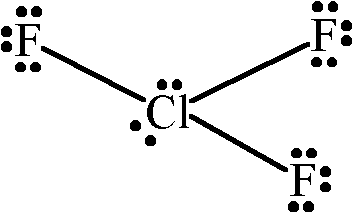
We can see that the central
