An HCl solution has a concentration of 3.26 * 10^-2 M. What is the pH of this solution?
1 Answer
Jun 14, 2016
The pH of this solution is 1.487.
Explanation:
Since HCl is a strong acid, it completely dissociates into its respective ions when placed in water. In this case HCl ionizes to produce
As soon as we know that we're dealing with a strong acid, the pH can be obtained directly from the concentration of
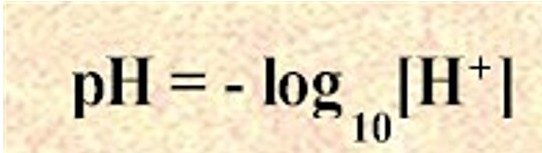 lpoli.50webs.com
lpoli.50webs.com
All we have to do is take the -log of the given concentration like so:

