Arrange KBr, CsI and SrS in order of increasing bond order?
1 Answer
#"BO"_"SrS" > "BO"_"KBr" > "BO"_"CsI"#
Well, they're all ionic compounds, so the only practical way to obtain the "bond order" is through the lattice energies.
LATTICE ENERGY STRENGTH TRENDS
Bond order, qualitatively speaking, is proportional to the bond strength. When considering ionic compounds, we expect the following typical trends:
- The smaller the ionic radii of the cation and anion, the stronger the bond.
- The smaller the difference in ionic radii between the cation and anion, the stronger the bond.
- The larger the charge magnitudes of the cation and anion, the stronger the bond. This is the predominant effect.
Increasing bond order is directly proportional to increasing lattice energy.
EXAMINING THE LATTICE ENERGY FACTORS
Now consider these ions on the periodic table:
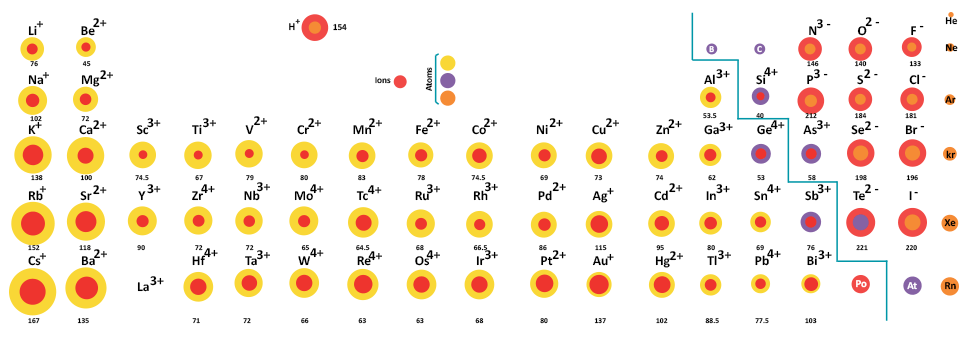
In order of smallest to largest ionic radii, we have:
#"SrS"# (#r_(A^(+)) = "132 pm"# ,#r_(X^(-)) = "184 pm"# )#"KBr"# (#r_(A^(+)) = "152 pm"# ,#r_(X^(-)) = "182 pm"# )#"CsI"# (#r_(A^(+)) = "181 pm"# ,#r_(X^(-)) = "220 pm"# )
In order of most similar to least similar ionic radii, we have:
#"KBr"# (#"152 pm"# vs.#"182 pm"# ,#Deltar_("ionic") = "30 pm"# )#"CsI"# (#"181 pm"# vs.#"220 pm"# ,#Deltar_("ionic") = "39 pm"# )#"SrS"# (#"132 pm"# vs.#"184 pm"# ,#Deltar_("ionic") = "52 pm"# )
And finally, in order of largest to smallest charge magnitude, we have:
#"SrS"# (#"A"^(2+), "X"^(2-)# )#"KBr"# and#"CsI"# (#"A"^(+), "X"^(-)# )
The charge magnitude affects the lattice energy the most by far, followed by the actual ionic radii.
FINAL ORDERING + DATA
The above three methods of comparison are all ordered by their effect on decreasing bond strength.
So, we expect the highest lattice energy to least lattice energy to be:
#"SrS"# (highest charge magnitude; large difference in ionic radii)#"KBr"# (smallest charge magnitude; most similar ionic radii; smallest ionic radii as a#pm1# ionic compound)#"CsI"# (smallest charge magnitude; less similar ionic radii than#"KBr"# ; larger ionic radii than#"KBr"# as a#pm1# ionic compound)
And to check, I have some data here:
#DeltaH_"lattice"("SrS") = ???# (cannot find)Comparable replacement:
#color(blue)(DeltaH_"lattice"("CaO") = -"3464 kJ/mol")#
Compare that to these:
#color(blue)(DeltaH_"lattice"("KBr") = -"688 kJ/mol")#
#color(blue)(DeltaH_"lattice"("CsI") = -"604 kJ/mol")#
So, we were correct.
The larger negative value we have for the lattice energy, the more energy released when the lattice was formed, and thus the stronger the lattice structure and the higher the bond order.

