Arrange the alkanes in each set in order of increasing boiling point.explain.(i) 2-methylheptane,octane, and 2,2,4-trimethylpentane.(ii)butane,octane and hexane?
1 Answer
Part (i): 2,2,4-trimethylpentane < 2-methylheptane < octane
Explanation:
These are all alkanes, so their boiling points will depend on the relative strengths of the London dispersion forces among the molecules.
Part (i)
The structures of the compounds are

2-methylheptane
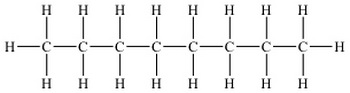
(from www.chem.ucla.edu)
octane
2,2,2-Trimethylpentane is an impossible structure.
I have substituted 2,2,4-trimethylpentane for your compound. Its structure is

2,2,4-trimethylpentane
The strength if London dispersion forces depends on how well the molecules can pack together.
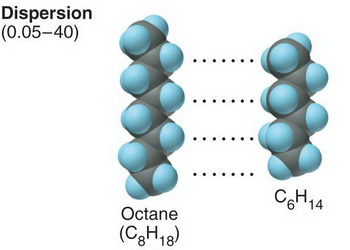
(from www.suggestkeyword.com)
The n-alkanes can best fit together and maximize their London dispersion forces.
Substituents will hinder the approach of other molecules and prevent them from getting close enough for strong attractions.
Their boiling points will be lower.
The order of boiling points should be
2,2,4-trimethylpentane < 2-methylheptane < octane
Their actual boiling points are 99 °C < 116 °C < 125 °C
Part (ii)
"We leave it as an exercise for the student" to answer Part (ii).
Hint: It has to do with the effect of chain length on the strength of London dispersion forces.

