At what temperature is water the most dense? Why is this the case with water, but not other substances?
1 Answer
As you remark, this is a very unusual property of water....
Explanation:
Solid ice, under normal pressure, is LESS dense than liquid water. And hence ice cubes (and ice bergs!) float....and the fact we find this familiar should not make us ignore what a HIGHLY unusual property this is. The solid phase SHOULD be more dense than the liquid phase; that water does not follow this rule can probably be attributed to hydrogen bonding.
For water's maximum density..we consult the data...
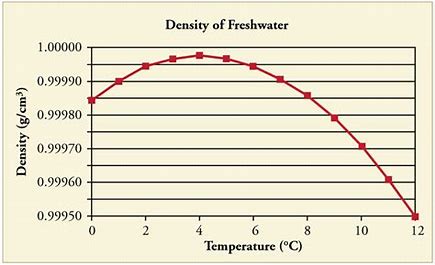
And from the graph, we find that water exhibits its maximum density under standard pressure at

