Also see here...
Bond order for #"NO"^+#
Order by bond length: #"NO"#, #"NO"^(+)#, #"NO"^(-)#
Is #"CO"# a Lewis acid?
#"O"_2# is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that #"CO"# is not (as it has zero unpaired electrons), but #"NO"# is (it has one unpaired electron).
Well, the MO diagram for #"O"_2# is:
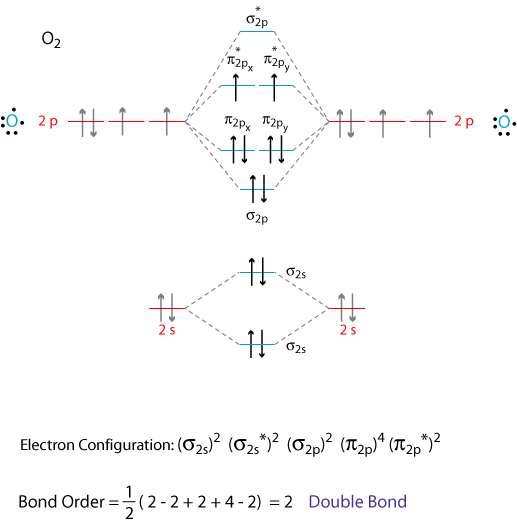
The bond order is already calculated in the diagram.
The bonding electrons are in the #sigma_(2s)#, #sigma_(2p)#, #pi_(2p_x)#, and #pi_(2p_y)# MOs, giving #2+2+2+2 = 8#.
The typo on the #sigma_(2s)^"*"# in the diagram should be corrected, and afterwards, the antibonding electrons are in the #sigma_(2s)^"*"#, #pi_(2p_x)^"*"#, and #pi_(2p_y)^"*"# MOs, giving #2+1+1 = 4#.
#"BO" = 1/2("bonding e"^(-) - "antibonding e"^(-))#
#= 1/2(2+2+2+2 - 2 - 1 - 1) = color(blue)(2)#
What we should comment on is that the bond order is supposed to be #2#. After all, it's a double bond...
#:ddot"O"=ddot"O":#
The MO diagram for #"CO"# is:
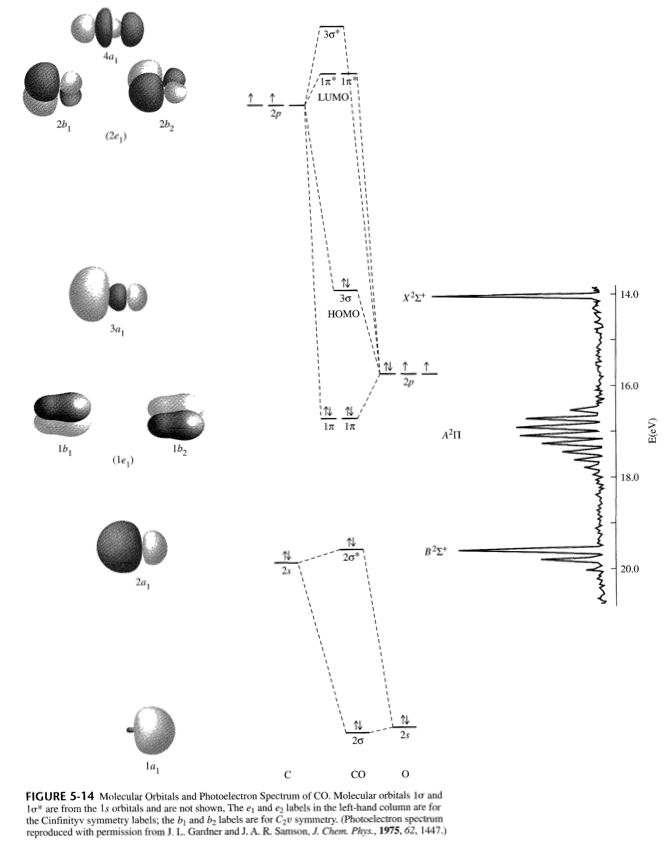
The bonding MOs are the #2sigma#, #1pi_x#, #1pi_y#, and #3sigma#, which gives #2+2+2+2 = 8# bonding electrons. The antibonding MO is the #2sigma^"*"#, which gives #2# antibonding electrons.
Hence, the bond order here is:
#"BO" = 1/2("bonding e"^(-) - "antibonding e"^(-))#
#= 1/2(2+2+2+2-2) = color(blue)(3)#
And this is sensible... #"CO"# has a triple bond...
#:"C"-="O":#
#"CO"# has #14# electrons, while #"NO"^(+)# has #14# electrons (gained one by #"C" -> "N"# and lost one by #"NO" -> "NO"^(+) + e^(-)#), so #"NO"^(+)# and #"CO"# are isoelectronic.
#:"C"-="O":#
#:"N"-="O":^(+)#
Hence, the bond order of #"NO"# is either #2.5# or #3.5#, depending on whether the last electron went into a bonding or antibonding MO.
The MO diagram of #"NO"# is:

The last electron is in fact in the #2b_1# antibonding MO, so the bond order of #"NO"# has decreased by #1/2# relative to #"NO"^(+)# or #"CO"#.
Therefore the bond order is #color(blue)(2.5)#. That corresponds to the Lewis structure of:
#:dot"N"=ddot"O":#
with a resonance hybrid structure of:
#""^((-0.5)):dot"N"stackrel("- -"color(white)(.))(=)dot"O":^((+0.5)#
And this indeed shows #2.5# bonds, as one electron is shared in a half-#pi#-bond.




