Can a titration be applied to determine the concentration Na3PO4(aq) and NaH2PO4(aq) in a solution mixture of the two salts using HCl and phenolphthalein and methyl orange indicators?
My attempt to answer the question:
Na2HPO4 (aq) is fully ionized in aqueous solution into two Na+ (aq) and one HPO42- ions. The solution mixture also contains NaH2PO4 which dissolves completely in water to produce one Na+ and one H2PO4- ions giving rise to high concentrations of H2PO4- and HPO42- ions and producing a buffer solution. This happens as the equivalence point of the first reaction is reached; assuming that number of moles of acid is equal to the number of moles of its salt. Phenolphthalein would not be suitable indicator because it only changes color in alkaline regions (8.2-10).
Halfway the third equivalence point a second buffer solution is produced corresponding to a pH of 2.12. This buffer solution is a mixture of undissociated phosphoric acid molecules and its conjugate base H2PO4- ; both species are present in equal concentrations. Using these observations, one can conclude that methyl orange indicator would not be a suitable indicator to use because it only changes color in pH values ranging from 3.2-4.4.
My attempt to answer the question:
Na2HPO4 (aq) is fully ionized in aqueous solution into two Na+ (aq) and one HPO42- ions. The solution mixture also contains NaH2PO4 which dissolves completely in water to produce one Na+ and one H2PO4- ions giving rise to high concentrations of H2PO4- and HPO42- ions and producing a buffer solution. This happens as the equivalence point of the first reaction is reached; assuming that number of moles of acid is equal to the number of moles of its salt. Phenolphthalein would not be suitable indicator because it only changes color in alkaline regions (8.2-10).
Halfway the third equivalence point a second buffer solution is produced corresponding to a pH of 2.12. This buffer solution is a mixture of undissociated phosphoric acid molecules and its conjugate base H2PO4- ; both species are present in equal concentrations. Using these observations, one can conclude that methyl orange indicator would not be a suitable indicator to use because it only changes color in pH values ranging from 3.2-4.4.
1 Answer
Yes, you can use a titration to determine the concentration if you use phenolphthalein indicator.
Explanation:
The equation for the equilibrium is
A mixture of
However, as you add
The pH will then change rapidly when you reach the first equivalence point.
Here is a titration curve for a salt like
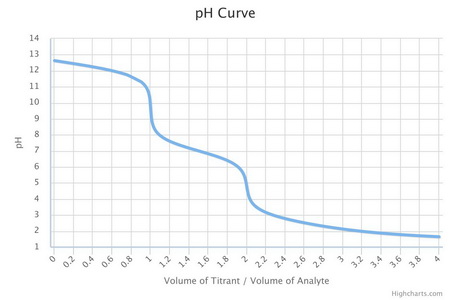
If you have a mixture of the two salts, your titration curve would start at about 0.5 on the volume axis with
As you add
Hence, you can easily recognize the end-point at pH 9.5 by using phenolphthalein
(

