Can anyone explain to me how to identify epimers and anomers in carbohydrates (sugars)? Also, state examples.
1 Answer
Epimers and anomers are both optical isomers that differ in the configuration at a single carbon atom, but there is a difference in their definitions.
Explanation:
Epimers
Epimers are optical isomers that differ in the configuration of a single carbon atom
For example, D-galactose and D-mannose are epimers of D-glucose.
 biochemnoob.files.wordpress.com
biochemnoob.files.wordpress.com
(from biochemnoob.wordpress.com)
D-Galactose is an epimer of D-glucose because the two sugars differ only in the configuration at
D-Mannose is an epimer of D-glucose because the two sugars differ only in the configuration at
Anomers
When a molecule such as glucose converts to a cyclic form, it generates a new chiral centre at
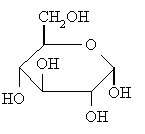 www.chem.ucalgary.ca
www.chem.ucalgary.ca
(from www.chem.ucalgary.ca)
The carbon atom that generates the new chiral centre (
Anomers are special cases — they are epimers that differ in configuration only at the anomeric carbon.
For example, α-D-glucose and β-D-glucose are anomers.
 wikispaces.psu.edu
wikispaces.psu.edu
(from wikispaces.psu.edu)
The α form has the anomeric
The β form has the anomeric
In D-fructose, the carbonyl group is at
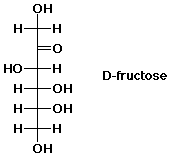 web.pdx.edu
web.pdx.edu
(fromweb.pdx.edu)
Here,
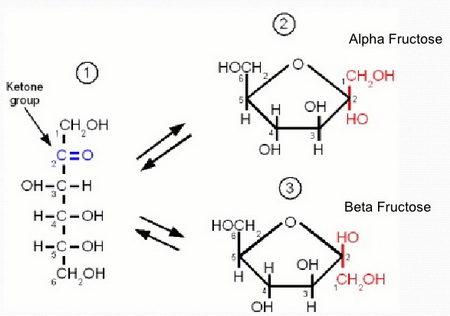 Fructose
Fructose
(From pinterest.com)
α-D-Fructofuranose and β-D-fructofuranose are anomers.

