Can someone solve question iv? (its the top one)
1 Answer
Latent heat required to be removed along with the energy needed to be released to bring down the temperature to
Explanation:
This happens because when the water at
This latent heat is required for the change in state of matter,because water at
So,on releasing that amount of energy,the kinetic energy of water molecule is reduced to such a state that they will get relatively stable and sit closer to make it a solid form i.e ice.
For,
That means
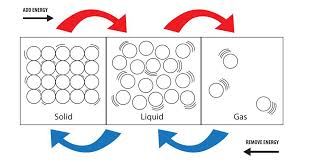
So,this amount of heat energy has to be released from the water which will require some time to cause the complete conversion.

