Consider Hydrogen ion in a box having one dimension, then find electron probability distribution?
1 Answer
These solutions are well-known and you should get to know what they look like. Here are the wave function
Also, on the side, we got that
#k = (npi)/L = sqrt((2mE)/ℏ^2)# ,
it follows that the energy levels are given by:
#color(blue)(E_n) = (ℏ^2k^2)/(2mL^2) = color(blue)((ℏ^2n^2pi^2)/(2mL^2))#
DISCLAIMER: LONG ANSWER!
A hydrogen cation (presumably
The Hamiltonian for such a scenario is:
#hatH = hatK + cancel(hatV)^(0)#
#= -ℏ^2/(2m) (del^2)/(delx^2)#
The box looks like:
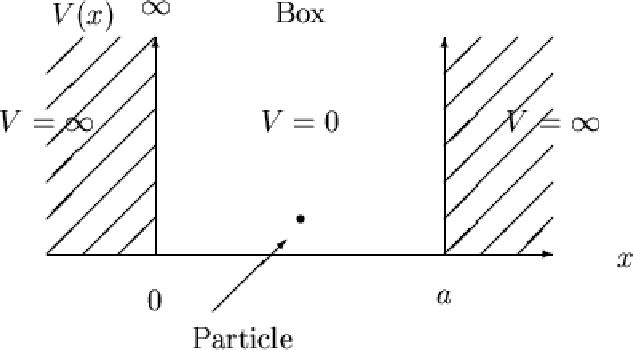
with boundary conditions
#V = {(0, x in (0,L)),(oo, x <= 0),(,x >= L):}#
The Schrodinger equation is then:
#hatHpsi = Epsi#
#=> -ℏ^2/(2m) (d^2psi)/(dx^2) = Epsi#
Rearrange to the standard form:
#(d^2psi)/(dx^2) + (2mE)/(ℏ^2)psi = 0#
Often we set the substitution
#(d^2psi)/(dx^2) + k^2psi = 0#
The general solution to this is assumed to be
#psi = e^(rx)#
and upon inserting it, we obtain the auxiliary equation:
#r^2 e^(rx) + k^2 e^(rx) = 0#
In the well,
#r = ik#
and we write a linear combination for
#psi = c_1e^(ikx) + c_2e^(-ikx)#
Using Euler's formula, we rewrite this in terms of real trig functions.
#psi = c_1(cos(kx) + isin(kx)) + c_2(cos(kx) - isin(kx))#
#= (c_1 + c_2)cos(kx) + (ic_1 - ic_2)sin(kx)#
Define
#psi = Acos(kx) + Bsin(kx)#
The boundary conditions state that since the potential goes to
#Acos(kcdot0) + Bsin(kcdot0) = Acos(kL) + Bsin(kL) = 0#
But since
#Bsin(kL) = 0#
And this is only when
Thus, since
#psi_n(x) = Bsin((npix)/L)#
And the probability distribution is then:
#psi_n^"*"(x)psi_n(x) = B^2 sin^2((npix)/L)#
A well-behaved wave function is normalized in its boundaries, so we say that
#int_(0)^(L) psi_n^"*"psi_ndx = 1#
From this we get the normalization constant.
#1 = B^2 int_(0)^(L) sin^2((npix)/L)dx#
Consider the following argument.
If
The identities
Therefore,
#color(blue)(psi_n^"*"(x)psi_n(x) = "Probability Distribution")#
#= color(blue)(2/L sin^2((npix)/L))#

