Could someone please explain (ii)* to me?
I don't get part ii), all of it is Greek to me TT I don't know why tertiary carbocations are more stable, how methyl groups stabilise charge, no idea what inductive effect or steric hindrance means. Could someone pls pls help me out here?


I don't get part ii), all of it is Greek to me TT I don't know why tertiary carbocations are more stable, how methyl groups stabilise charge, no idea what inductive effect or steric hindrance means. Could someone pls pls help me out here?




1 Answer
ISOMER 1: n-bromobutane
The primary (
#"H"_3"C"-"CH"_2-"CH"_2-stackrel(delta^+)(stackrel("*")"C")"H"_2-stackrel(delta^-)"Br"#
You said that you are using
Due to the low steric hindrance around that carbon (no non-H substituents are on
Therefore, the nucleophile and substrate will both participate in a reaction that has no intermediate, giving a rate law of:
#r(t) = k["OH"^(-)]["C"_4"H"_9"Br"]#
The reason why an intermediate doesn't form is that primary carbocations are very unstable.
The electron density from a
The more alkyl groups you have surrounding the
Instead of an intermediate, a transition state forms, which is simply going to look halfway between the reactant and the product. It will be a trigonal bipyramidal geometry around the central carbon!
#" "" "" "" "" "" "" "" "color(white)(.)"Br"#
#" "" "" "" "" "" "" "" "color(white)(.)vdots#
#"H"_3"C"-"CH"_2-"CH"_2-stackrel("*")"C"^(delta^+)"H"_2#
#" "" "" "" "" "" "" "" "color(white)(.)vdots#
#" "" "" "" "" "" "" "color(white)(....):ddot"O":^(delta^(-))#
#" "" "" "" "" "" "" "" "" ""|"#
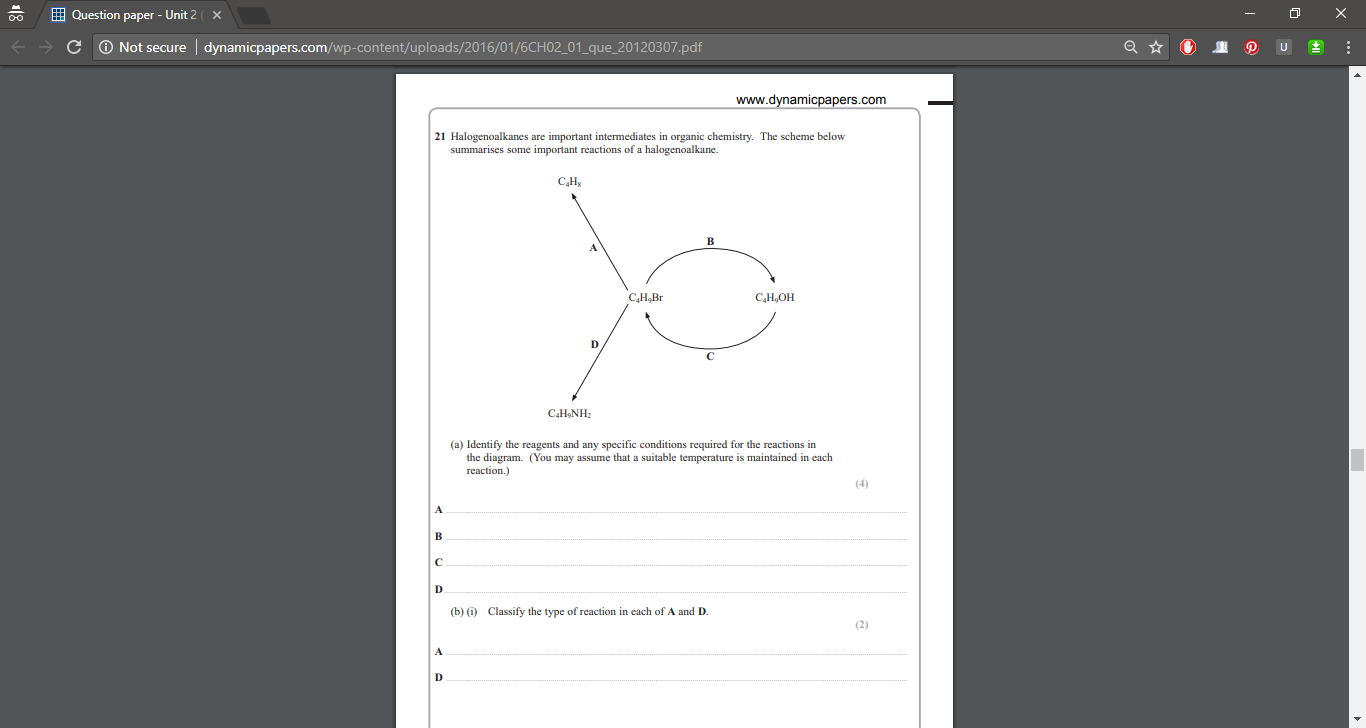
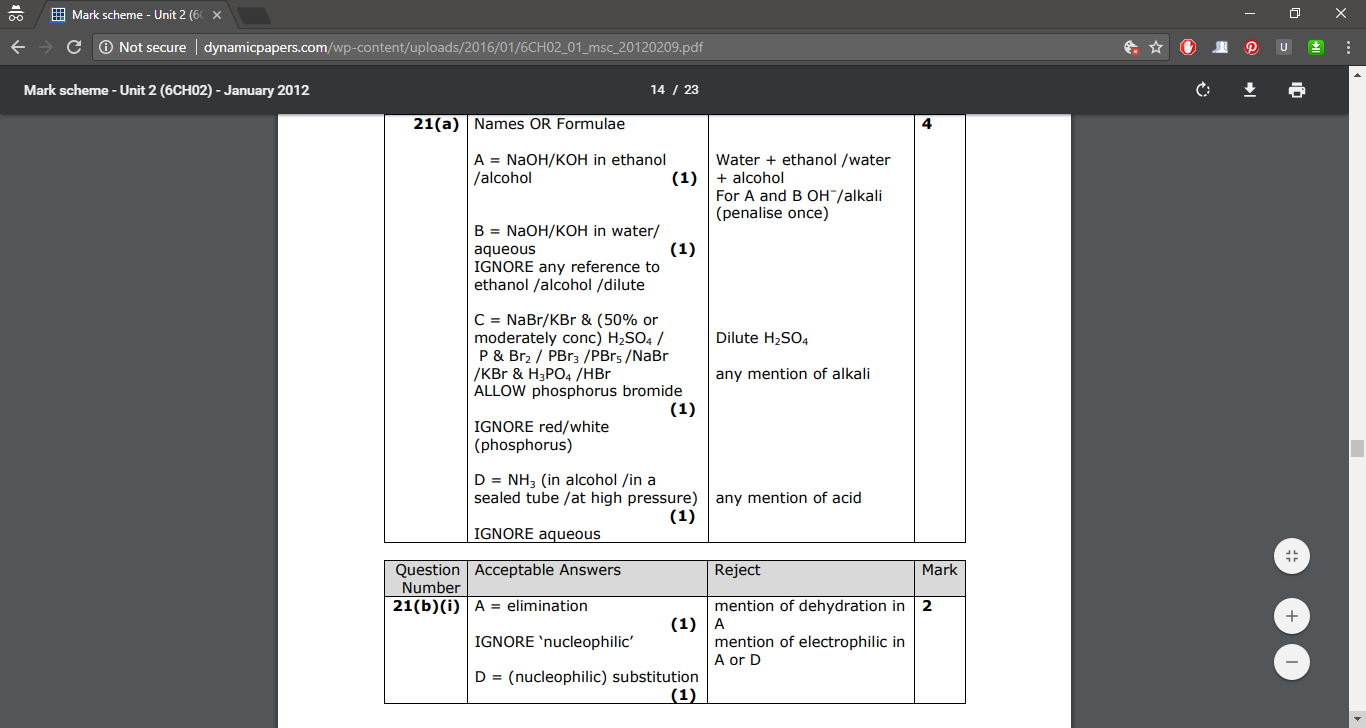
#" "" "" "" "" "" "" "color(white)(.....)"H"#
Of course, you should draw it with the proper bond angles. Be sure to include the partial charges.
ISOMER 2: tert-butyl bromide
You can tell then that tert-butyl bromide (
#" "" "" ""CH"_3#
#" "" "" ""|"#
#"H"_3"C"-"C"-"Br"#
#" "" "" ""|"#
#" "" "" ""CH"_3#
It has a tertiary (
Hence, a first-order substitution (
#r(t) = k_1[("CH"_3)_3"CBr"]# (where the first step is slow)
while the intermediate looks like this:
#" "" "" ""CH"_3#
#" "" "" ""|"#
#"H"_3"C"-"C"^(+)#
#" "" "" ""|"#
#" "" "" ""CH"_3#
(Of course, you should be drawing this with the proper bond angles!)
This is stabilized by the three
This stabilizing effect is much stronger than in a primary carbocation.
[Being planar, the intermediate will lead to a racemic mixture of

