Could someone please group these bonds under Polar and Non-Polar and also under hydrophobic and hydrophilic? C=O, C-H, O-P, and C-C
1 Answer
Polar, nonpolar, polar, nonpolar.
Explanation:
You can determine the polarity of a bond by looking at the electronegativity of the atoms that make up tha bond.
More specifically, you need to examine the difference in electronegativity between the atoms that form the covalent bond.
Electronegativity is a measure of how good an atom that's part of a bond is at attracting the bonding electrons towards itself.
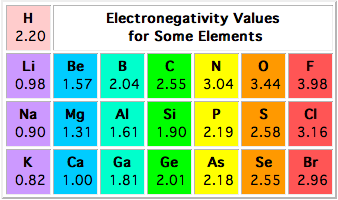 http://www.f.u-tokyo.ac.jp/~fukuyama/interactive_trial/virtual%20textbook/electronegativity/electronegativity.html
http://www.f.u-tokyo.ac.jp/~fukuyama/interactive_trial/virtual%20textbook/electronegativity/electronegativity.html
A polar covalent bond is formed when one of the two atoms is more electronegative than the other.
When this happens, the bonding electrons will spend more time surrounding the more electronegative atom, which will develop a partial negative charge.
The less electronegative atom will be "electron deprived", so it will develop a partial positive charge.
The difference in electronegativity,
#Delta_(EN) < 0.5 -># nonpolar covalent bond
#0.5 < Delta_(EN) < 1.7 -> # polar covalent bond
#Delta_(EN)> 1.7 -># predominantly ionic bond
So, if you pair oxygen,
#Delta_EN = 3.44 - 2.55 = 0.89 -># polar covalent bond
The bond between carbon and hydrogen is considered nonpolar because you have
#Delta_(EN) = 2.55 - 2.20 = 0.35#
The bond between phosphorus and oxygen is polar covalent
#Delta_(EN) = 3.44 - 2.19 = 1.25#
The bond between two identical atoms will always be nonpolar covalent, because you essentially have
#Delta_(EN) = 2.55 - 2.55 = 0#

