Covalent bonds are directional in nature. Explain?
1 Answer
In essence, the first covalent bond (a
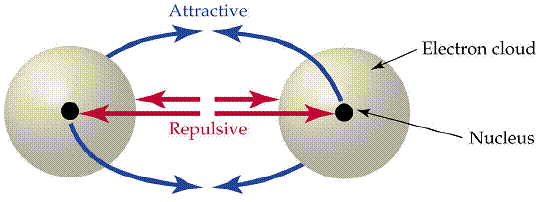
Another perspective is that the first covalent bond formed (a
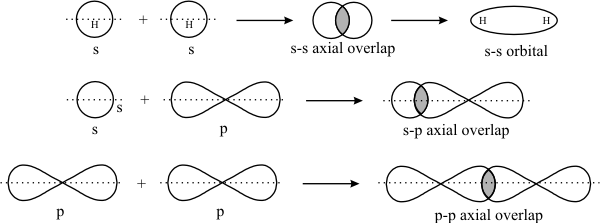
Either way, forming the first covalent bond requires two free atoms to approach each other in a straight line, which is therefore directional.
Even if we discussed the second covalent bond made, which is a
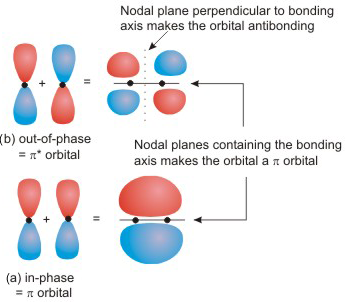
This is directional in the sense that the overlap requires the orbitals to remain aligned in the same manner (which they will) as the atoms approach each other, and that shall be accomplished as long as they approach each other in a straight line.

