Determine the final pH of a 0.200M triprotic phosphoric acid solution?
Socratic, Let's work together to find the answer to this question. There is no specific answer and it is open to intepretation.
Socratic, Let's work together to find the answer to this question. There is no specific answer and it is open to intepretation.
2 Answers
The pH of triprotic phosphoric acid 0.200 M will be 1.41
Explanation:

The second part of the solution

Explanation:
There is not enough information given in the question to determine the pH at the 3rd equivalence point so I will assume we are starting with 10.00 ml of
The pH can be monitored with a pH meter.
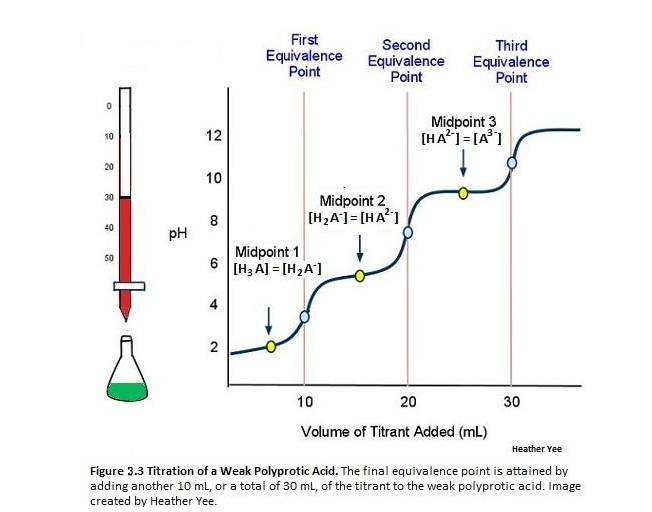
The curve given in the question appears to be a generic one for a triprotic acid. The phosphoric acid curve differs slightly:

As you can see there is no rapid change in pH at the 3rd equivalence point.
This is due to the effect of the auto - ionisation of water.
Phosphoric acid is triprotic and has 3 dissociations:
You can estimate the
If you consider
Rearranging the expression for
So
This is marked on the graph . You can read this off at 0.5 equivalents which, in our case, would be when 10 ml of base are added. This gives
To get the pH at the first equivalence point again, you can read this off the graph but a good way of doing this is to split the difference between
The same reasoning can be applied to the 2nd equivalence point.
At the 3rd equivalence point all the protons have been lost and we have a solution of sodium phosphate:
From the graph you can read off the pH when 3 equivalents of base has been added which, in our case will be 30 ml of 0.2 M NaOH.
This gives a pH of just over 12.
We can calculate this as follows:
The number of moles of
From the equation the number of moles of
The total volume of the solution in the flask after titration =
The phosphate ion is quite basic and is hydrolysed by water:
For which
To find
We can do this using:
Now we can set up an ICE table based on concentrations in
Because of the large value of
This can be solved using the quadratic formula, which I won't go into here. Ignoring the -ve root this gives:
So
Since
Then



