Determine the geometries using vsepr theory .State the approximate bond angle(s) in each molecule and determine whether each molecule is polar or non polar a)BCl3 b)PCl5 c)NCl3 d)SO3 e)XeF4 f)HBF2?
1 Answer
See below
Explanation:
a)
We leave it as an exercise for the student to do this one.
b)
Another exercise, but here's a link to the structure of
c)
(i) Lewis structure
https://cluster13-files.instructure.com/courses/986898/files/31116480/course%20files/003%20Chapter%209%20Chemical%20Bonding%20I%3A%20%20Lewis%20Theory/Electron%20Dot%20Structures/07048%20bbluejpg
(from usflearn.instructure.com)This is an
"AX"_3"E"AX3E molecule.(ii) Shape — Trigonal pyramid
http://file2.answcdn.com/answ-cld/image/upload/h_320,c_fill,g_face:center,q_60,f_jpg/v1401098305/ql7oh2p4szvm3yphhcp5png
(from www.answers.com)(iii) Bond angles — slightly less than 109.5°
(iv) Polarity — polar
(d)
(i) Lewis structure
(from gallery4share.com)This is an
"AX"_3AX3 system.(ii) Shape — Trigonal planar
http://glencoe.mcgraw-hill.com/sites/dl/free/007874637x/514757/trigonal_planarjpg
(from www.suggestkeyword.com)(iii) Bond angles — 120°
(iv) Polarity — nonpolar
All bond dipoles cancel, therefore
e)
(i) Lewis structure
http://images.tutorvista.com/cms/images/101/lewis-dot-structure-of-xef4png
(from chemistry.tutorvista.com)This is an
"AX"_4"E"_2AX4E2 structure.(ii) Shape — square planar
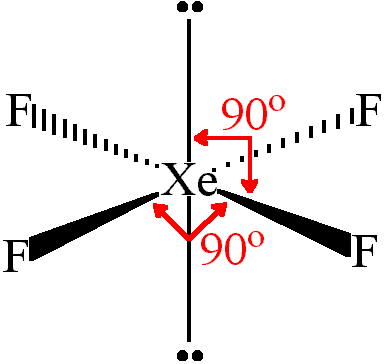
(iii) Bond angles — 90°
(iv) Polarity — nonpolar
f)
(i) Lewis structure
(from www.uwplatt.edu)This is an
"AX"_3AX3 structure.(ii) Shape — Trigonal planar
(iii) Bond angles — Approximately 120°.
(iv) Polarity — polar



