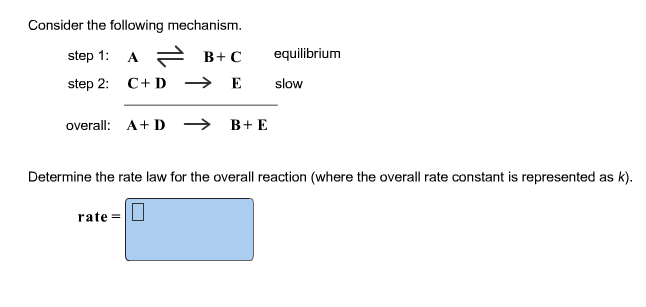
Determine the rate law for the overall reaction (where the overall rate constant is represented as k)?
1 Answer
I would get that
#A stackrel(k_1" ")rightleftharpoons B + C# #" "" "# (#"fast equilibrium"# )
#""^(" "k_(-1))#
#ul(C + D stackrel(k_2" ")(->) E" "" ")# (#"slow"# )
#A + D stackrel(k" ")(->) B + E#
We begin with the notion that the slow step is the rate-determining step. From that, the initial form of the rate law is:
#r(t) = k_2[C][D]# where
#k_2# is the rate constant for the forward second step.
However,
#K = k_1/k_(-1) = ([B][C])/([A])#
is the equilibrium constant for the first reaction step. Therefore:
#[C] = k_1/k_(-1) ([A])/([B])#
and we can substitute back into the rate law to get:
#r(t) ~~ (k_2k_1)/(k_(-1)[B]) [A][D]#
We would use
In the expected notation,
#color(blue)(r(t) ~~ k[A][D])# ,where
#k = (k_2k_1)/(k_(-1)[B])# .

