Do aromatic hydrocarbons have at least one planar ring with delocalized p electrons?
1 Answer
Jun 13, 2015
No, but aromatic hydrocarbons have at least one planar ring with delocalized π electrons.
Explanation:
There are no atomic
The π orbitals must also contain
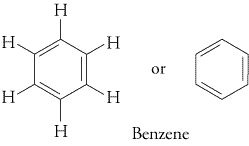
The best known aromatic hydrocarbon is benzene.
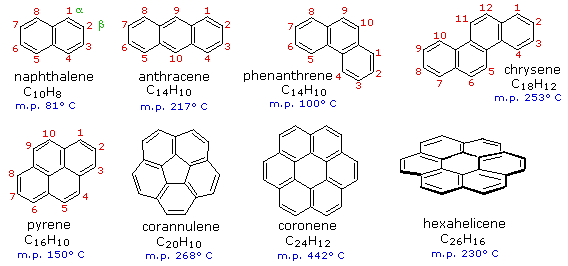
Below are the structures of aromatic hydrocarbons containing more than one ring.