Does a negative #DeltaH# correspond to an endothermic or exothermic process?
1 Answer
Dec 5, 2016
-
Explanation:
When the value of
Consider the graphic below:
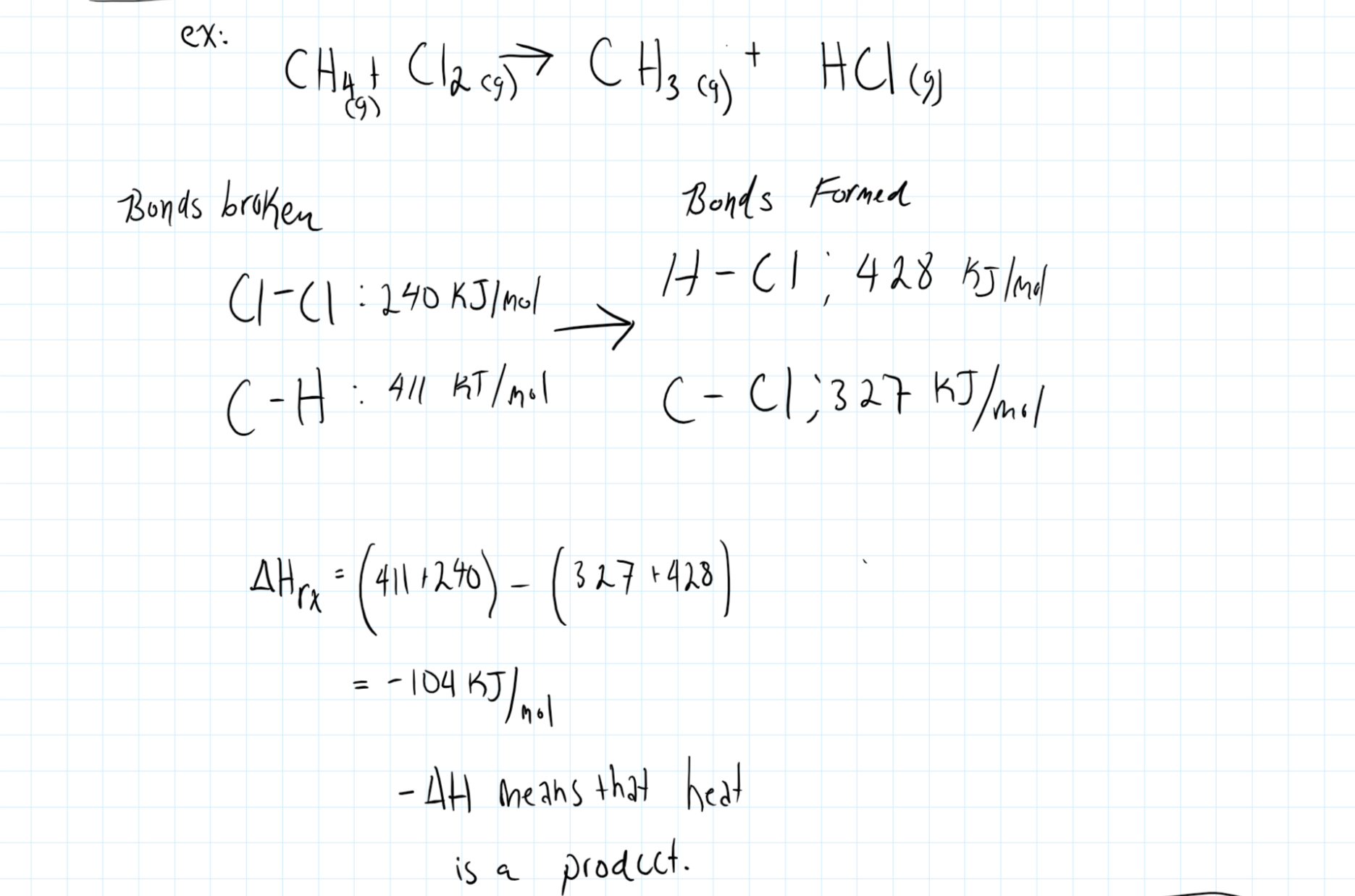
As side note, you should consider that the formation of bonds are what result in a net release of energy from the system, and that bond formation is completely driven by the electronic properties of the species.

