Does anyone know how to work this Celsius equation?
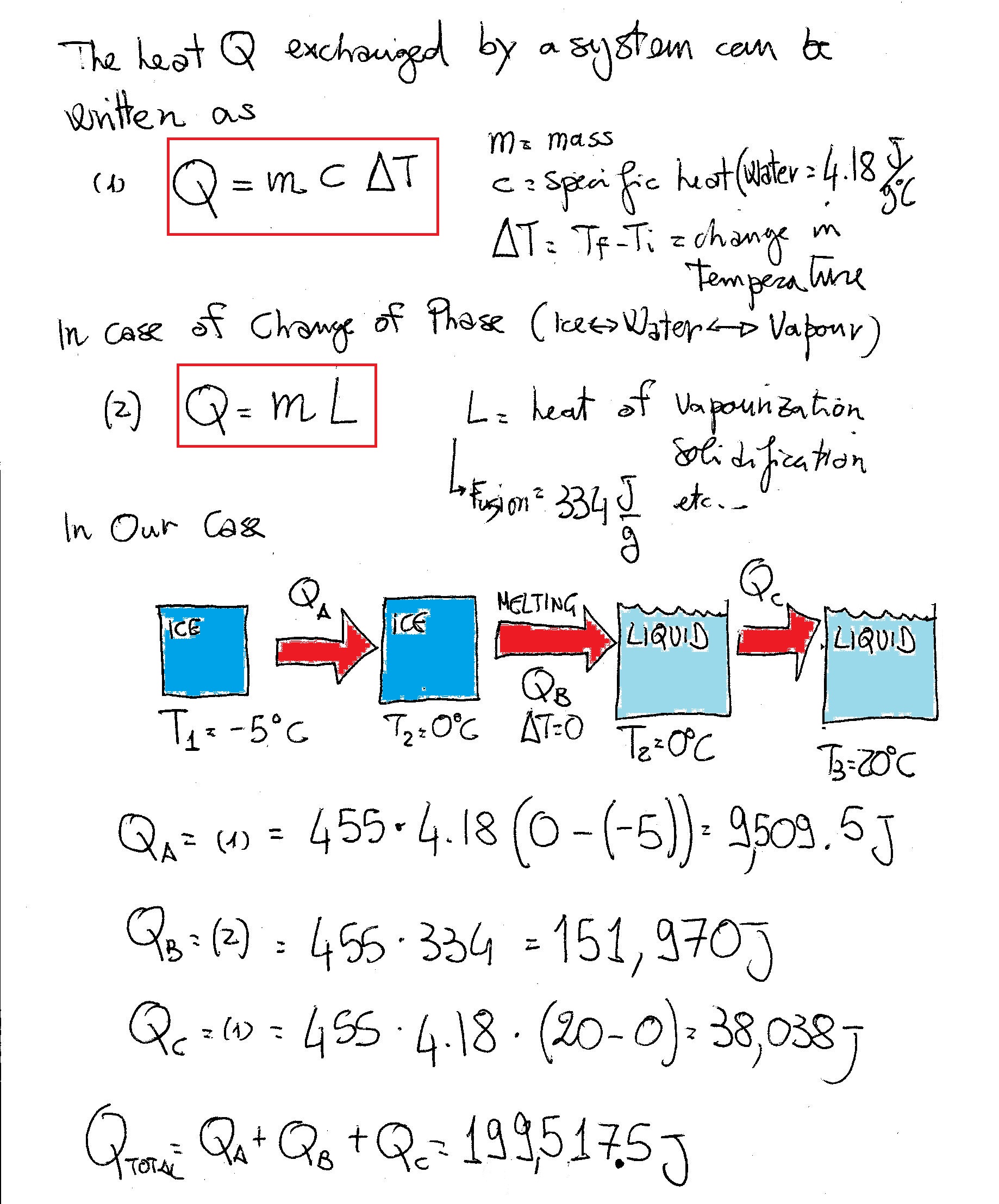
How much heat is necessary to change 455 g of ice at -5°C to water at 20°C?
How much heat is necessary to change 455 g of ice at -5°C to water at 20°C?
2 Answers
Explanation:
In this process first we need to convert the ice at
Next,we need to supply the required latent heat to change its state from ice to equal amount of water at
Now,latent heat required for melting of ice =
So,for
Now,again we have to supply heat energy to convert water at
So,heat energy required for the entire process is the sum of the above
I got almost
Explanation:
Have a look: