Does #"CH"_3"COCOCH"_3# show tautomerism?
1 Answer
Yes, it will show keto-enol tautomerism.
Explanation:
Keto-enol tautomerism
Keto-enol tautomerism is a rapid chemical equilibrium between a keto and an enol form of a carbonyl compound.
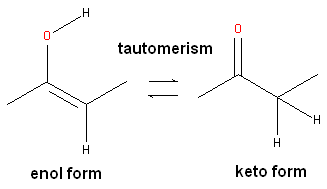
The keto form is usually the more stable, but some structural features can favour the enol.
There must be an
The α-hydrogen ends up on the oxygen atom and a
Butane-2,3-dione
Butane-2,3-dione has sp³ α-hydrogens at
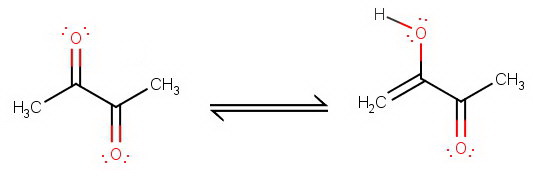
The keto form is almost certainly the most stable form.
I would expect little stabilization of the enol by intramolecular hydrogen bonding because the two

