Does pressure affect Gibbs free energy?
1 Answer
Sep 9, 2017
Absolutely. It is particularly significant for gases.
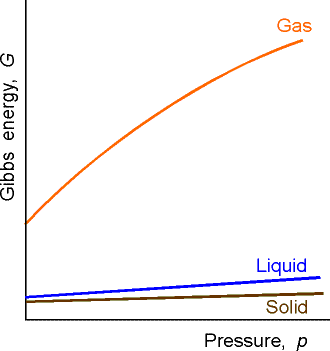
The Gibbs' free energy is a function of temperature and pressure. Its Maxwell Relation shows:
#dG = -SdT + VdP#
The
At constant temperature and pressure, the Gibbs' free energy for systems closed to mass and energy transfer goes to zero.
This is exemplified in ordinary spontaneous phase transitions, where one has constant temperature and is letting the phase transition occur naturally at a constant atmospheric pressure. Thus,
#DeltaG_"tr" = 0 = DeltaH_"tr" - T_"tr"DeltaS_"tr"#
and at a given phase transition of this sort,
#DeltaS_"tr" = (DeltaH_"tr")/T_"tr"#

