Does the VSEPR theory predict that XeF2 is linear?
1 Answer
The VSEPR theory predicts that XeF₂ is linear.
We must first draw the Lewis structure for XeF₂.

This tells us that there are five electron regions (Steric Number = 5) about the central carbon atom. They are the three lone pairs and the two Xe-F bonds.
The VSEPR model states that the electron regions around an atom spread out to make each region is as far from the others as possible.
Here is a diagram of various VSEPR shapes.
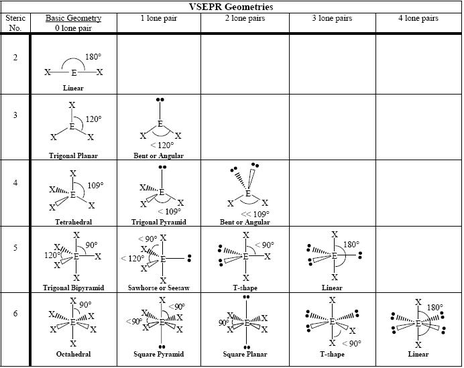
We see that when the SN = 5, the electrons arrange themselves at the corners of a trigonal bipyramid.
The lone pairs take up the most space, so they occupy the equatorial positions.
The C-F sigma bonds occupy the axial positions.
The shape of the molecule refers only to the arrangement of the bonds.
The bonds are at angles of 180° from each other, so XeF₂ is a linear molecule.

