Electrochemistry question? (Voltaic Cell)
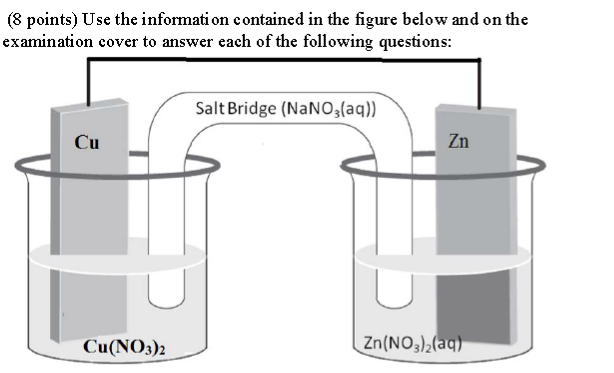
A: Which electrode is the cathode in the reaction above?
B: Give the half reaction occurring at each of the electrodes below. Make sure it shows the direction the reaction actually proceeds in the cell above.
C: Write a balanced chemical equation describing the total reaction associated with the electrochemical cell.
D: What is the EMF of the cell above if it is operated under standard state conditions?


A: Which electrode is the cathode in the reaction above?
B: Give the half reaction occurring at each of the electrodes below. Make sure it shows the direction the reaction actually proceeds in the cell above.
C: Write a balanced chemical equation describing the total reaction associated with the electrochemical cell.
D: What is the EMF of the cell above if it is operated under standard state conditions?
1 Answer
I will break it down for you so you can understand how to look at these types of questions.
Explanation:
This is a Galvanic cell. Galvanic cells are spontaneous meaning they have a
You have solid
- Since we know that
"Cu"^(+2) ions are more likely to be reduced to become"Cu"(s) , thecolor(red)("Cu electrode is labeled as the cathode") while thecolor(red)("Zn electrode is labeled as the anode") , where oxidation occurs. (remember the memory aid AN OX, RED CAT)
- If reduction is occurring at the
"Cu" cathode (where we know the solution of"Cu(NO3)"_2 ionizes to give off"Cu"^(+2) cations and"NO"_3^(-1) anions), this means"Cu"^(+2) ions will gain2e^- and will deposit onto the"Cu" electrode. The half reaction is the following for the left half cell:
- In the right half cell, the
"Zn" electrode (which is the anode) will lose2e^- and"Zn"^(+2) ions will fall out into the solution which already has"Zn"^(+2) cations and"NO"_3^(-1) ions from the zinc nitrate solution. The half reaction in the right cell is the following:
- If we combine the two half reactions, we get
- Okay. We have established that at the
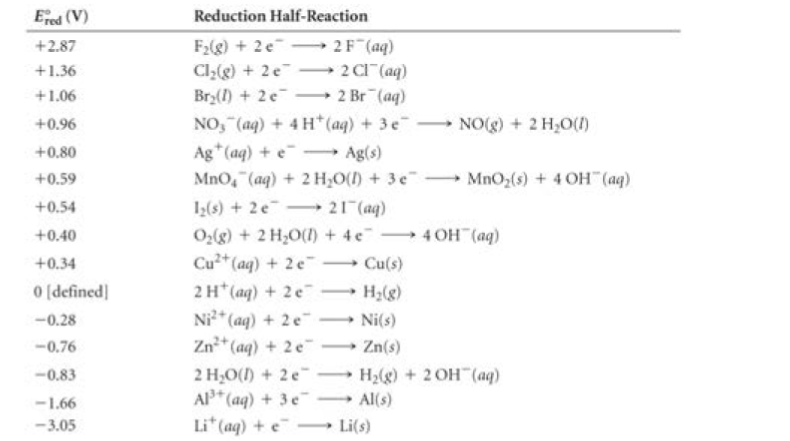
"Cu" half cell, reduction will occur and at the"Zn" half cell. oxidation will occur. To find the EMF of the cell, the following equation is going to be used.
Since we were provided
- Use the equation to plug in and solve:

