Electronic configuration of bromine ?
2 Answers
The bromine atom...?
Explanation:
Well, for bromine,
...or
The electron configuration of Bromine is
This can be shortened to
Explanation:
Use a chart such as the one below to fill the subshells in order of the diagonal lines. 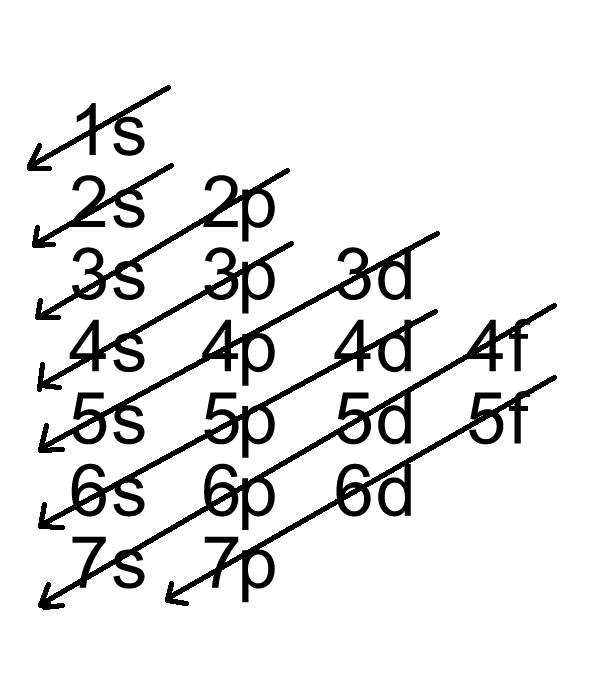
The angular momentum quantum number,
An


