Electronic valence molecular orbital configuration of #"O"_2^(+)#?
Would be very grateful for an explanation, i've never seen this before. cheers

Would be very grateful for an explanation, i've never seen this before. cheers
1 Answer
#(sigma_(2s))^2(sigma_(2s)^"*")^2(sigma_(2p_z))^2(pi_(2p_x))^2(pi_(2p_y))^2(pi_(2p_x)^"*")^1#
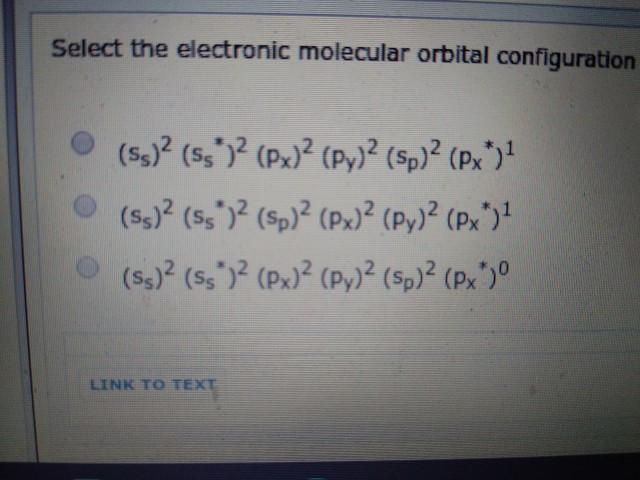
Your first answer option is for
Your second answer option is for
Your third answer option is for
You should be able to draw the MO diagram for
You'll need the molecular orbital (MO) diagram of

Each oxygen contributes
- Two
#2s# orbitals combine to give a#sigma_(2s)# bonding and#sigma_(2s)^"*"# antibonding MO. - Two
#2p_x# orbitals combine to give a#pi_(2p_x)# bonding and#pi_(2p_x)^"*"# antibonding orbital. These are the same energy as the#pi_(2p_y)# counterparts. - Two
#2p_y# orbitals combine to give a#pi_(2p_y)# bonding and#pi_(2p_y)^"*"# antibonding orbital. These are the same energy as the#pi_(2p_x)# counterparts. - Two
#2p_z# orbitals combine to give a#sigma_(2p_z)# bonding and#sigma_(2p_z)^"*"# antibonding MO.
Lastly,

For
#(sigma_(2s))^2(sigma_(2s)^"*")^2(sigma_(2p_z))^2(pi_(2p_x))^2(pi_(2p_y))^2(pi_(2p_x)^"*")^1(pi_(2p_y)^"*")^1#
In your notation,
#(s_(s))^2(s_(s)^"*")^2(s_(p))^2(p_(x))^2(p_(y))^2(p_(x)^"*")^1(p_(y)^"*")^1#
If you want
#color(blue)((sigma_(2s))^2(sigma_(2s)^"*")^2(sigma_(2p_z))^2(pi_(2p_x))^2(pi_(2p_y))^2(pi_(2p_x)^"*")^1)#
In your notation,
#color(blue)((s_(s))^2(s_(s)^"*")^2(s_(p))^2(p_(x))^2(p_(y))^2(p_(x)^"*")^1)#

