Explain how the rate of vaporization of a liquid affected with increase in temperature of the liquid?
1 Answer
The rate of vaporization increases as the liquid temperature increases. Evaporation occurs when vaporization is still nonspontaneous, i.e. is not thermodynamically favorable.
The natural equilibrium state at which the rate of vaporization equals the rate of condensation is achieved at the normal boiling point, i.e.
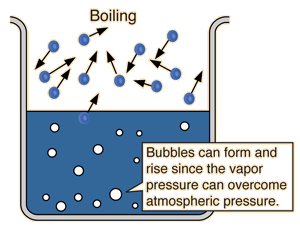
At this temperature, the vapor pressure exerted upwards by the evolving gas equals the atmospheric pressure exerted downwards onto the surface of the solution.
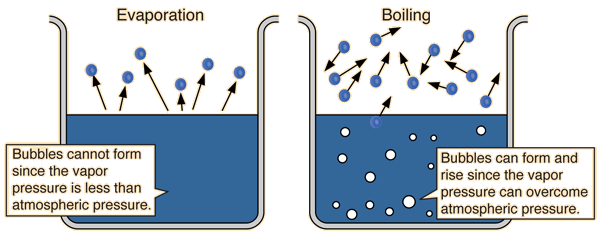
BEFORE this temperature is reached, i.e.
The vapor pressure is generally low at this point.
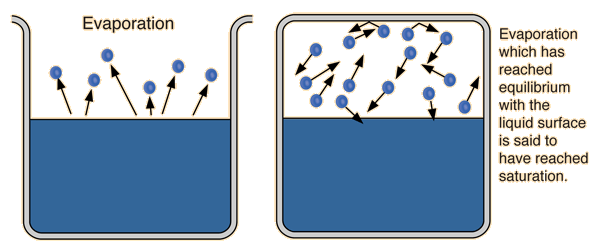
As
At this point, vaporization and condensation occur at the same rate.

