Explain why #"Li"_2"CO"_3# decomposes at a lower temperature, whereas #"Na"_2"CO"_3# decomposes at a higher temperature?
1 Answer
First, write out what the decomposition reaction is; it may help:
#"M"_2"CO"_3(s) -> "M"_2"O"(s) + "CO"_2(g)#
Now, recall what
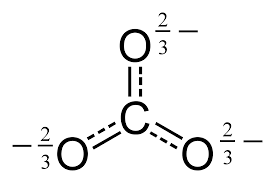
Clearly, it has a negative charge. The alkali metals are known as hard acids (from HSAB theory), because:
- They have high charge density (relative to same-row elements to their right).
- Their typical ionic form is a cation,
#"M"^(+)# . - They polarize electron density towards themselves, because it is
#delta^(-)# while the hard acids are#delta^(+)# . - Their ionic radii are quite small.
So, when they are near negative ions, they polarize the
This weakens the
Note that
#"Na"^(+): "Larger cationic radius"#
#-> "Less tightly-packed charge density"# #("softer hard acid")#
#-> "More polarizable by carbonate ion"#
#-> "Less polarizing towards carbonate ion"#
#-> "C"-"O"# #"bond on carbonate ion less weakened"# #("electrons more evenly shared")#
#-> "The sodium carbonate solid is therefore"# #"harder to decompose"#
#-> color(blue)("Higher decomposition temperature for Na"_2"CO"_3)#

