How are the graphs for:?
>Boyle's Law
>Pressure Law
_>Charles Law
>Boyle's Law
>Pressure Law
_>Charles Law
1 Answer
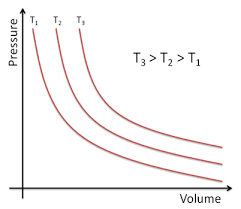
Boyle's Law : At constant temperature(
or,
or,
this is an equation of rectangular hyperbola
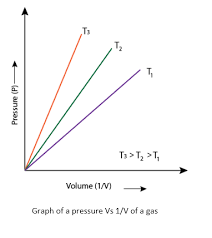
And,
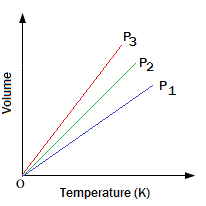
Charle's Law: At constant pressure (
or,
this an equation of straight line passing through origin.
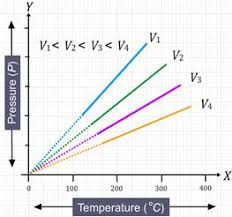
Gay-Lussac's Law or Pressure Law: At constant volume(
or,
so,this is also an equation of straight line passing through origin.