How can benzene be converted into acetophenone?
1 Answer
Jan 27, 2016
Think of the differences between the two compounds.
The former is a benzene ring. The latter is a benzene ring plus an acyl group. Let's put that into graphic form.
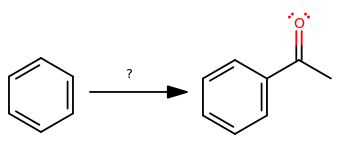
Okay, so how do we get there? Suppose we break the bond that was just made and use that idea to figure out what could have happened.
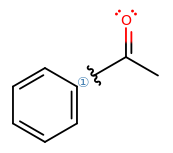
From here...
- What is required for a bond to form? A nucleophilic attack. From whom? Benzene. Benzene initates the bond formation because (with some coercion via a catalyst, namely
"AlCl"_3 ) benzene can be willing to donate electrons from itspi bonds. - Why? Because the target spot on the molecule was electrophilic, or electron-poor.
- What made it electrophilic? The oxygen withdrawing electrons due to the
~1.0 electronegativity difference between oxygen and carbon. So this electrophilic "spot" must be the carbonyl carbon. - How did that spot become attractive to benzene? Because it became particularly electropositive.
- How? A functional group was coerced to leave by a catalyst. But benzene REALLY loves being aromatic. The functional group would have to leave a
(+) charge in order to be a prime, reactive target for attack, so the functional group must have taken its electrons with it. - A catalyst like
"AlCl"_3 , which is a Lewis acid with an emptyp orbital, can convince an acyl chloride to donate"Cl"^(-) straight off of the compound. There we go!
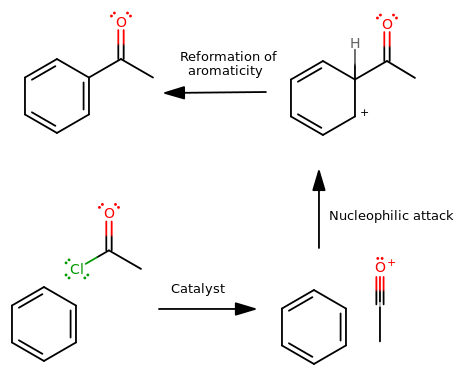
CHALLENGE: Can you fill in the mechanistic arrows? Hint: You should be able to find this in your book. It's the Friedel-Crafts Acylation.

