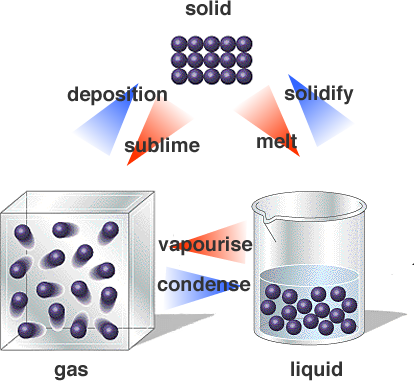
How can changes of state be explained by the particle theory?
1 Answer
As particles gain enough energy, they can escape from the attractions of their neighbours and go into a more fluid state.
All particles attract each other. At the same time, all molecules are in constant motion. Their kinetic energies drive them apart.
At low temperatures, the kinetic energy of the molecules is low. The particles cannot move fast enough to get away from the attractions of their neighbours. They are “glued” in place so that they can vibrate only a little from side to side in a crystalline arrangement. The molecules are in the solid state.
At higher temperatures, the kinetic energy of the particles is higher. The particles have enough energy to slide past their neighbours. They do not have enough energy to escape the attractions of their neighbours. The system is in the liquid state.
At high temperatures, the kinetic energy of the particles is high. The particles can escape the attractions of their neighbours and move at will within the container. The system is in the gas state.