How can enantiomers be distinguished?
2 Answers
How? Well, principally by the use of models.
Explanation:
In any undergrad exam in organic chemistry, a set of models will be permitted. Of course, you have to be able to use the models, and also be able to represent the 3D model on the page.
If you have (correctly) represented a stereocentre on the printed page (or on a model!), say you have
So, not only do have to use the models, you also have to be able to represent them graphically. This is a non-trivial exercise. Check past exams for the types of questions you will be asked.
You can distinguish enantiomers by (a) making models, (b) assigning
Explanation:
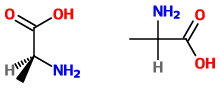
A typical homework or exam question goes something like: "Are the following compounds identical, enantiomers, diastereomers, or structural isomers?"
(a) Making models
When to use them — when you have lots of time!
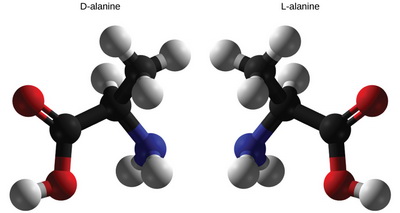
(From biowiki.ucdavis.edu)
Models are great when you are just learning the concepts, but it takes time to build them.
In a test or exam situation, you are typically stressed for time.
Use them only when you have answered the other questions and have time to build the models.
Often, you will not have enough "atoms" to build the models, anyway.
(b) Assigning
This usually requires no manipulations of the structures.
In the example question, the priorities of the groups are:
#"NH"_2 = bb1# #"COOH" = bb2# #"CH"_3 = bb3# #"H" = bb4#
In the wedge-dash structure, the
In the Fischer projection, the horizontal bonds are wedges, and the vertical bonds are dashes.
The
The compounds have opposite configurations, so they are enantiomers.
(c) Looking for mirror images
Here, you will have to convert one structure to the same format as the other.
Let's convert the Fischer projection to wedge-dash.
We rotate it 90 ° counterclockwise to bring the
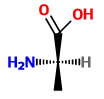
We tilt the
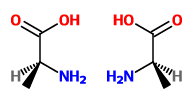
We see that the molecules are nonsuperimposable mirror images (enantiomers).


