How can I convert 3-methylhexane C_3−C_4 bond from bond line notation to Newman projection?
1 Answer
May 18, 2015
Step 1. Draw the structure of 3-methylhexane.
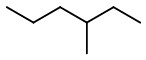 Bond line
Bond line
Step 2. Convert to a wedge-dash structure at C-3 and C-4.
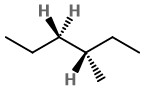
Step 3. Identify the groups on C-2 and C-3.
The groups on C-2 are CH₃CH₂, CH₃, and H. Those on C-3 are CH₃CH₂, H, and H.
Step 4. Draw a template for a Newman projection.
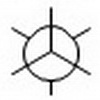
Step 5. Attach the groups to the carbons of your template.
View the molecule from the lower right.
The groups on C-2 go on the front carbon atom. Put a CH₃CH₂ group on the top, a CH₃ group on the right, and an H atom on the left.
The groups on C-3 go on the back carbon. The bulky CH₂CH₃ group goes on the bottom, and the two H atoms go on the other bonds.
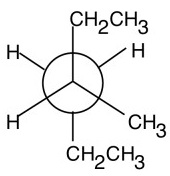
And you have a Newman projection for 3-methylhexane.

