Bond Line Notation to Newman Projection
Key Questions
-
Answer:
You convert the bond-line structure to a wedge-dash structure. Then you convert the wedge-dash structure to a Newman projection.
Explanation:
The bond line structure of ethane is

The wedge-dash structures for the staggered and eclipsed conformations are:
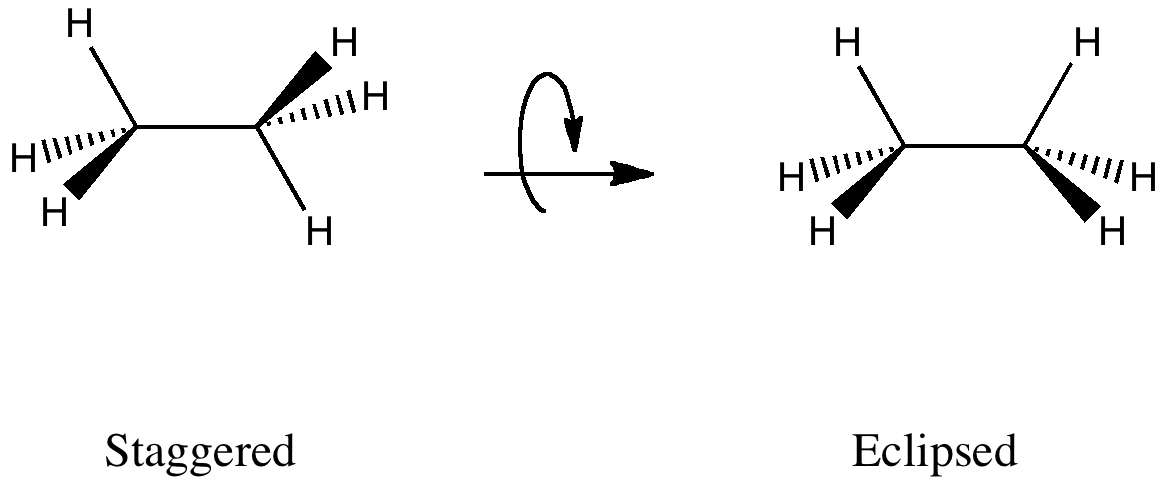
Here's how to convert the wedge-dash structures to Newman Projections.
Staggered Ethane
Step 1. View the molecule along the C1-C2 axis with your eye at the left-hand end.
The closest bonds make an inverted Y, so you will choose that template for your projection.
Step 2. Place the groups onto your template.
All groups on the carbon atoms are H atoms.

And you have your staggered Newman projection for ethane.
Eclipsed Ethane
Step 1. Draw two eclipsed Newman templates.
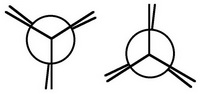
Step 2. View the molecule along the C1-C2 axis with your eye at the left-hand end.
The closest bonds make an inverted Y, so you will choose the second template for your projection.
Step 3. Place the groups onto your template.
All groups on the carbon atoms are H atoms.
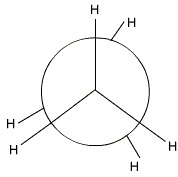
And you have your eclipsed Newman projection for ethane.
-
A bond-line notation gives no stereochemical information, but here's how to convert a wedge-dash notation to a Newman projection.
PROBLEM
Convert the following wedge-dash structure of 2-chloro-3-methylpentane to a Newman projection through the C2-C3 bond.

Step 1. Draw two Newman projection templates.
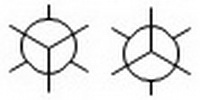
- Draw two circles.
- Put a dot in the centre of each. These represent C-2. C-3 is hiding behind the circles.
- On one circle, draw a Y with the spokes radiating from the dot at 120° angles. On the other circle, draw an inverted Y. These represent the bonds from C-2.
- Draw lines coming off each circle that bisect the angles of the Y. These represent the bonds from C-3.
You will choose one of these templates as the basis for your projection.
Step 2. View the molecule along the C2-C3 axis with your eye closest to C2.
Your eye will be at the lower left of the image.
The closest bonds make an inverted Y, so you will choose the second template for your projection.
Step 3. Place the groups onto your template.
- The groups on C-2 go on the front carbon, The groups on C-3 go on the back carbon.
- The "normal" groups go on the vertical lines: CH₃ in front; CH₂CH₃ behind.
- The wedged groups go on the right hand side: H in front; CH₃ in back.
- The dashed groups go on the left hand side: Cl in front; H in back.

And you have your Newman projection.