How can I convert percent concentration to molarity?
1 Answer
Here's one way to do it.
Explanation:
Example
Concentrated hydrochloric acid is usually available at a concentration of 37.7% by mass. The density of the solution is 1.19 g/mL. What is its molar concentration?
Solution
Step 1. Calculate the mass of 1 L of solution.
Step 2. Calculate the mass of
Step 3. Calculate the moles of
Step 4. Calculate the molarity of the HCl.
Here's a summary of the types of calculations we were using above
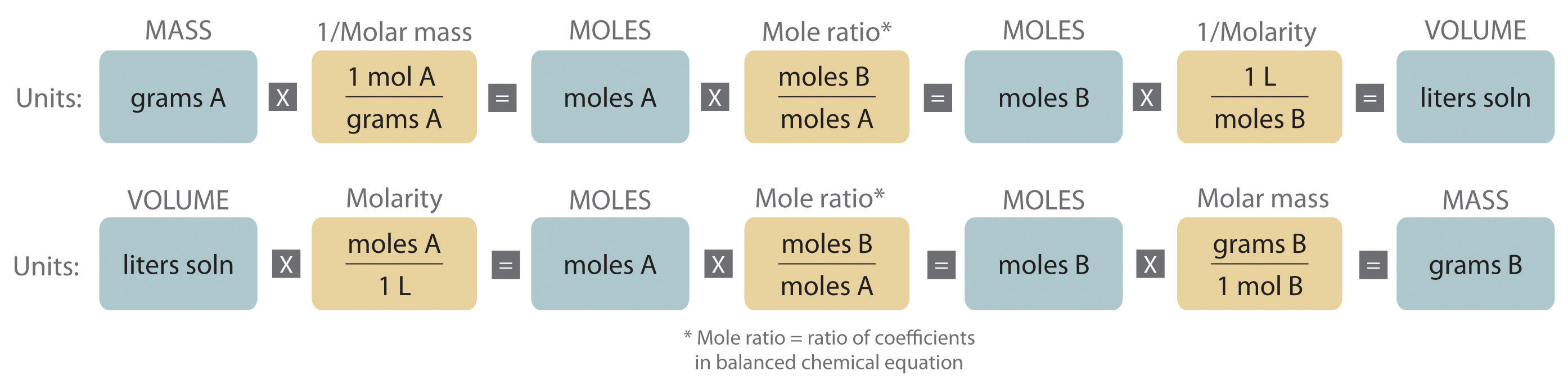
And here's a video about converting mass % to molarity.


