How can I convert S-1-fluoro-2-chloropropane to a Fisher projection?
1 Answer
May 18, 2015
Step 1. Draw the bond-line structure of 1-fluoro-2-chloropropane.
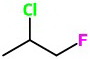
Step 2. Convert this to a wedge-dash structure.
(a) Start by making the Cl bond a wedge. We have a 50 % chance of getting it right.
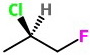
(b) Assign configurations. This is the
(c) Draw the correct structure. Convert the Cl bond to a dash.
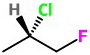
Step 3. Draw the skeleton Fischer projection
To convert this to a Fischer projection, draw a vertical line of three carbon atoms, with C-1 at the top and a cross at C-2.
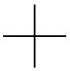
Step 4. Attach the F atom to C-1 and the H and the Cl atoms to the correct sides of the cross.
We must look at the molecule from the upper left. This puts Cl on the left and H on the right.
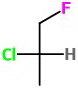
And we have our structure.

