Introduction to Fisher Projections
Key Questions
-
Fischer projections are best used to represent the straight-chain structures of monosaccharides.
A Fischer projection is a drawing of a 3D molecule as a flat structure. The chiral carbons are represented by crossed lines.
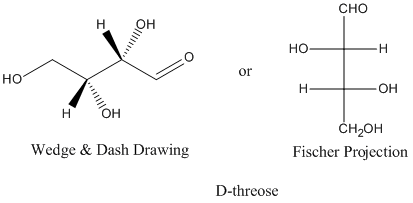
In addition to carbohydrates, it is useful for other molecules that have two or more chiral carbons.

Fischer projections are useful for differentiating between enantiomers, diastereomers, and meso compounds.

It is often easier to see an internal plane of symmetry in a Fischer projection.
-
A Fischer projection is a way of showing a three dimensional structure in two dimensions. It shows the absolute configuration at chiral centres.
In a Fischer projection, the longest chain is drawn vertically.
The four bonds to a chiral carbon make a cross, with the carbon atom at the intersection of the horizontal and vertical lines.
The two horizontal bonds are pointing toward the viewer. The two vertical bonds are directed away from the viewer.
